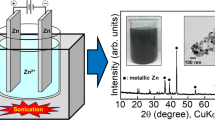
Abstract
During the electrowinning of zinc in a mixture electrolyte of zinc sulfate and ammonium sulfate, the effects of current density, Zn2+ mass concentration and electrolytic additives on the morphology and current efficiency of Zn powders were evaluated. Finer Zn powders were obtained at high current density and low Zn2+ mass concentration. However, the current efficiency decreased. Adding a proper amount of sodium dodecyl sulfate generated spherical Zn powders, and adding ethanol produced dendritic ones.




Similar content being viewed by others
REFERENCES
Hou, X.G., Wang, S., and Wang, Y.M., Preparation and characterization of nanometer ZnO powder, Powder Metall. Ind. (in Chin.), 2004, vol. 14, pp. 10–13.
Tang, Y.Q. and Tang, A.Y., Study on preparation of high grade Zinc from Zinc hydrometallurgy system, Hunan Nonferrous Met. (in Chin.), 2014, vol. 30, pp. 34–37.
Zhou, H.J., Wang, S.L., Deng, B., and Chen, X.L., Preparation and characterization of nanoflake zinc particles by vibration milling, Mater. Mech. Eng. (in Chin.), 2005, vol. 29, pp. 62–68.
Chen, W.M., Wan, X.H., and Sun, Y.Y., Study on technology of producing Zinc powders by gas atomization, Mater. Rev., 2000, vol. 14, pp. 59–61.
Wang, A.P., Liu, K., Wang, Y.J., Guo, L., Yang, Y.P., and Liang, T.B., Preparation of super fine Zinc dust by distillation-condensation technology, Nonferrous Met. (in Chin.), 2008, vol. 60, pp. 46–49.
Wang, P., Zhao, F.X., and Zhang, Z.Z., Preparation of ultrafine zinc powders by dc arc plasma evaporation method, Chin. J. Nonferrous Met. (in Chin.), vol. 21, pp. 2236–2241.
Yasumasa, I., Wei, X., Divyaraj, D., Dan, S., and Sanjoy, B., An indicator of zinc morphology transition in flowing alkaline electrolyte, J. Power Sources, 2012, vol. 211, pp. 119–128.
Li, Q., Zhao, Y.C., and Zhang, C.L., Influence of cetyltrimethylammonium bromide and sodium lauryl sulfate on production of zinc powders by alkaline electrowinning, Russ. J. Nonferrous Met., 2014, vol. 55, pp. 65–72.
Hossein Kamran, H., Davood, M., and Mohammad Hadi, S., Production of zinc powder from Co–Zn plant residue using selective alkaline leaching followed by electrowinning, Physicochem. Probl. Mi, 2015, vol. 51, pp. 411–425.
Ostanina, T.N., Rudoy, V.M., Nikitin, V.S., Darintseva, A.B., and Demakov, S.L., Change in the physical characteristics of the dendritic zinc deposits in the stationary and pulsating electrolysis, J. Electroanal. Chem., 2017, vol. 784, pp. 13–24.
Zhu, Y.C. and Qian, Y.T., Solution-phase synthesis of nanomaterials at low temperature, Sci. China Phys. Mech., 2009, vol. 52, pp. 13–20.
Li, L., Liu, J.T., and Ren, Z.J., Crystallization behavior of disproportionately high and low molecular weight PEO blends, Chin. J. Polym. Sci., 2014, vol. 32, pp. 1199–1209.
Wang, H.J., Feng, H.P., Wang, X.C., Du, Q.C., and Yan, C., Crystallization kinetics and morphology of poly(vinylidene fluoride)/poly(ethylene adipate) blends, Chin. J. Polym. Sci., 2015, vol. 33, pp. 349–361.
Xiao, G.Y., Lu, Y.P., Zhu, R.F., Xu, W.H., and Jiao, Y., Fabrication of hydroxyapatite microspheres with poor crystallinity using a novel flame-drying method, T. Nonferrous Metal. Soc., 2012, vol. 22, pp. s169–s171.
Gu, W.M., Liu, C.Q., Tang, J., Liu, R.Y., Yang, H., and Hu, J.G., Improving zinc electrodeposition in ammoniacal electrolytes with the saturated dissolved methyltrioctylammonium chloride, Hydrometallurgy, 2018, vol. 175, pp. 43–51.
Yuan, L., Ding, Z.Y., Liu, S.J., Shu, W.F., and He, Y.N., Effects of additives on zinc electrodeposition from alkaline zincate solution, T. Nonferrous Metal Soc., 2017, vol. 27, pp. 1656–1664.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (51304082).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
About this article
Cite this article
Rong Liang Zhang, Huang, A.D., Guo, H. et al. Preparation of Zinc Powders by Acid Electrowinning. Russ. J. Non-ferrous Metals 60, 173–178 (2019). https://doi.org/10.3103/S1067821219020160
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1067821219020160