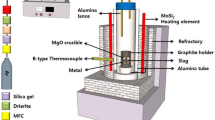
Abstract
High-chrome steel billet with excellent mechanical properties and corrosion resistance is widely used in the production of components for heavy machinery and power equipment. Electroslag remelting is an effective and widely used technology for the production of such billet. If this technology is introduced in the production chain, highly uniform metallurgical characteristics (chemical composition, structure, content of nonmetallic inclusions, etc.) of the steel and hence excellent mechanical properties of the steel products may be ensured by regulating the refining and solidification processes. Effective refining of high-chrome steel in electroslag remelting depends on correct selection of the slag and the maintenance of the optimal redox potential, since one role of the chromium and other elements present in the slag is to transport oxygen from the gas phase to the liquid metal. On the basis of the electron structure of the slag systems, the influence of the degree of oxidation of the slag and the equilibrium oxygen partial pressure \({{P}_{{{{{\text{O}}}_{2}}}}}\) on the oxidative state of the chromium in slags of type ANF-1, ANF-6, and ANF-29, which are widely used in Russia, may be assessed. The dependence of the concentration ratio Cr3+/Cr2+ on the temperature, degree of oxidation, and optimal basicity of the slag is established. A thermodynamic model is proposed for the variation in the oxidative state of chromium in the slag as a function of its degree of oxidation. Calculation results are compared with experimental data for slag systems at 1873 K. It is shown that the mean oxidative state of the chromium decreases with increase in temperature and with decrease in oxygen partial pressure and optical basicity of the slag. The presence of fluorine in the slag affects the ratio Cr3+/Cr2+. With decrease in oxygen partial pressure from 10–4 to 10–12 Pa at 1873 K, the mean oxidative state of chromium in fluoride–oxide slags decreases from +3 to +2. A formula for estimating the ratio Cr3+/Cr2+ in fluoride–oxide slags is proposed, taking account of the temperature and oxidation of the slag.



Similar content being viewed by others
REFERENCES
Kern, T.-U., Scarlin, B., Zeiler, G., et al., The European COST536 project for the development of new high temperature rotor materials, Proc. 17th Int. Forgemasters Meetings, Santander, 2008, no. 1081 p. 2.
Utkina, K.N., Balikoev, A.G., Levkov, L.Ya., Dub, V.S., et al., Principal technology of production of a new nanostructured corrosion-resistant duplex steel, 19‑ya Konferentsiya molodykh spetsialistov po yadernym energeticheskim ustanovkam (19th Conference of Young Specialists in Nuclear Power Plants), Podolsk: Gidro-Press, 2017, pp. 351–359.
Mitchell, A., Reyes-Carmona, F., and Wei, C.-H., Deoxidation in the electroslag process, Proc. 39th Electric Furnace Conf., Houston, 1981, pp. 103–107.
Reitz, J., Maurischat, M., and Friedrich, B., Optimized control of slag chemistry for the electroslag remelting of large size ingots, Proc. 17th Int. Forgemasters Meetings, Santander, 2008, pp. 28–36.
Hernandez-Morales, B. and Mitchell, A., Review of mathematical models of fluid flow, heat transfer and mass transfer in electroslag remelting process, Ironmaking Steelmaking, 1999, vol. 26, no. 6, pp. 423–438.
Ponomarenko, A.G. and Inozemtseva, E.N., Valence of metals in oxide and salt melts, Materialy 4-i Vsesoyuz. konferentsiya po stroeniyu i svoistvam metallicheskikh i shlakovykh rasplavov (Proc. 4th All-Union Conf. on the Structure and Properties of Metallic and Slag Melts), Kiev: Naukova Dumka, 1980, part 3, pp. 67–70.
Pavlov, A.V., Physicochemical properties of polyvalent elements in melts and development of energy-resource-saving metallurgical technologies, Extended Abstract of Doctoral (Eng.) Dissertation, Moscow, 2002.
Bartie, N.J., The effects of temperature, slag chemistry, and oxygen partial pressure on the behavior of chromium oxide in melter slags, MSc Thesis, Stellenbosch: Univ. of Stellenbosch, 2004.
Jahanshahi, S., Sun, S., and Zhang, L., Recent developments in physico-chemical characterization and modeling of ferroalloy slag systems, Proc. 10th Int. Ferroalloys Congr. “Infacon X,” Cape Town, 2004, pp. 316–332.
Mikelsons, J., Degree of oxidation of iron in SaO–SiO2–FeOn slag melts as a function of the oxygen partial pressure of the gas phase, Arch. Eisenhuttenwesen, 1982, vol. 53, no. 6, pp. 251–265.
Khrapko, S.A., Thermodynamic model of the metal-slag system for automatic control systems and machine experiments to optimize the steelmaking process, Extended Abstract of Cand. Sci. (Eng.) Dissertation, Donetsk, 1990.
Mitchell, A. and Etienne, M., Oxidative losses of low levels of titanium during electroslag remelting, Proc. Second Int. Symp. on Electroslag Remelting Technology, Pittsburgh, 1969, part 2.
Biele, H., Pateisky, G., and Fleischer, H.J., The reactions of titanium and silicon with Al2O3–CaO–CaF2 slags in the ESR process, J. Vac. Sci. Technol., 1972, vol. 9, no. 6, pp. 1318–1321.
Levkov, L.Ya., Theory and practical control of physical-chemical and thermophysical processes in electroslag remelting determining the quality of critical products, Extended Abstract of Doctoral (Eng.) Dissertation, Moscow, 2016.
Okoukoni, P.I., Development of CAD elements for steel melting technology, Extended Abstract of Cand. Sci. (Eng.) Dissertation, Donetsk, 1993.
Holappa, L. and Xiao, Y., Slags in ferroalloys production—review of present knowledge, J. South Afr. Inst. Min. Metall., 2004, vol. 104, no. 7, pp. 429–437.
Schwerdtfeger, K. and Mirzayousef-Jadid, A., Redox equilibria of transition metals in silicate melts, Proc. Belton Memorial Symp, Sydney, Australia, Warrendale, PA: Iron Steel Soc., 2000, pp. 108–119.
Mohanty, A.K. and Kay, D.A.R., Activity of chromic oxide in the CaF2–CaO–Cr2O3 and the CaF2–Al2O3–Cr2O3 systems, Metall. Mater. Trans. B, 1975, vol. 6, pp. 159–166.
Wang, L. and Seetharaman, S., Experimental studies on the sulphide capacities of CaO–SiO2–CrOx slags, Metall. Mater. Trans. B, 2010, vol. 41, no. 2, pp. 367–373.
Yan, B., Zhang, J., and Song, Q., Thermodynamic behaviour of transition metal (Cr, Ti, Nb, V) oxides in molten slags, Proc. VIII Int. Conf. on Molten Slags “Molten 2009,” Concepción: Univ. de Concepción, 2009, ch. 1, pp. 309-317.
Morita, K. and Sano, N., Activity of chromium oxide in CaO–SiO2 based slags at 1873 K, VII Int. Conf. on Molten Slags Fluxes and Salts, Cape Town: South Afr. Inst. Min. Metall., 2004, pp. 113–117.
Morita, K., Inoue, A., Takayama, N., and Sano, N., The solubility of MgO–Cr2O3 in MgO–Al2O3–SiO2–CaO slag at 1600°C under reducing conditions, Tetsu-to-Hagane, 1988, vol. 74, no. 6, pp. 999–1005.
Pauling, L., The Nature of the Chemical Bond, Ithaca, NY: Cornell Univ. Press, 1967.
Mendeleev 0.4.3 documentation. http://mendeleev.readthedocs.io/en/stable/data.html#electronegativity. Accessed March 12, 2018.
Li, K. and Xue, D., Estimation of electronegativity values of elements in different valence states, J. Phys. Chem. A, 2006, vol. 110, no. 39, pp. 11332–11337.
Cherkasov, A.R., Galkin, V.I., Zueva, E.M., and Cherkasov, R.A., The concept of electronegativity: the current state of the problem, Russ. Chem. Rev., 1998, vol. 67, no. 5, pp. 375–392.
Wegman, D.D., Investigation into critical parameters which determine the oxygen refining capability of the slag during electroslag remelting of alloy 718, MSc Thesis, Bethlehem, PA: Lehigh Univ., 1993.
Lakomskii, V.V. and Grigorenko, G.M., Approach to evaluation of the basicity of slag melt in gas-slag-metal system, Sovrem. Elektrometall., 2009, no. 2, pp. 48–49.
Povolotskii, D.Ya., Fiziko-khimicheskie osnovy protsessov proizvodstva stali: Uchebnoe posobie dlya vuzov (Physicochemical Foundations of Steel Production Processes: A Manual for higher Education Institutions), Chelyabinsk: Yuzhno-Ural. Gos. Univ., 2006.
Rudnenko, T.B., Ponomarenko, A.G., Inozemtseva, E.N., et al., Thermodynamic evaluation of the distribution of elements between slag and metal phases in the process of ESR, Probl. Spets. Elektrometall., 1987, no. 4, pp. 15–21.
Klechkovskii, V.M., Raspredelenie atomnykh elektronov i pravilo posledovatel’nogo zapolneniya (n + l)-grupp (Distribution of Atomic Electrons and the Rule of Gradual Filling of (n + l)-Groups]. Moscow: Atomizdat, 1968.
Konovalov, Yu.V., Statisticheskoe modelirovanie s ispol’zovaniem regressionnogo analiza: Metodicheskie ukazaniya k vypolneniyu kursovoi raboty po distsipline “Komp’yuternoe i statisticheskoe modelirovanie” (Statistical Modeling Using Regression Analysis: Methodical Instructions for the Course Work on the Discipline “Computer and Statistical Modeling”), Moscow: Mosk. Gos. Tekh. Univ. im. N.E. Baumana, 2013.
Pei, W. and Wijk, O., Experimental study on the activity of chromium oxide in the CaO–SiO2–Al2O3–MgOsat–CrOx slag, Scand. J. Metall., 1994, vol. 23, pp. 228–235.
Morita, K., Mori, M., Sano, N., et al., Activity of chromium oxide and phase relations for the CaO–SiO2–CrOx system at 1873 K under moderately reducing conditions, Steel Res., 1999, vol. 70, nos. 8–9, pp. 319–324.
ACKNOWLEDGMENTS
Financial support was provided by the Russian Ministry of Education and Science to promote the “Development of methods of controlling the physical, chemical, and structural uniformity of ingots in cyclic electroslag remelting and also a competitive resource-saving manufacturing technology for billet applicable at power plants, nuclear plants, and the oil and gas industry” (identifier RFMEFI57916X0134).
G.I. Matytsina and Zh.K. Kashirina of JSC “RPA “CNIITMASH” participated in this work.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by Bernard Gilbert
About this article
Cite this article
Levkov, L.Y., Shurygin, D.A., Dub, V.S. et al. Oxidation of Chromium in Oxide–Fluoride Slags for Electroslag Remelting. Steel Transl. 48, 766–772 (2018). https://doi.org/10.3103/S0967091218120069
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0967091218120069