More Information
Submitted: September 21, 2022 | Approved: September 27, 2022 | Published: September 28, 2022
How to cite this article: Tan CC, Yeo PHL, Mahalakshmi R, Omar AB, Whye Ng CL, et al. Shoulder recovery for head and neck cancer patients after unilateral neck dissection: a pilot exploratory study. J Nov Physiother Rehabil. 2022; 6: 011-017.
DOI: 10.29328/journal.jnpr.1001045
Copyright License: © 2022 Tan CC, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Head and neck cancer; Neck dissection; Shoulder disability; Physical therapy; Rehabilitation QOL
Shoulder recovery for head and neck cancer patients after unilateral neck dissection: a pilot exploratory study
Celia Ia Choo Tan1*, Pauline Hui Ling Yeo2, Mahalakshmi Rangabashyam3,4, Aisyah Binte Omar1, Cindy Li Whye Ng1, Rehena Sultana5, Kevin Netto6,7, Meng Ai Png8, Rahul Nagadia3-5, Gerald Ci An Tay3,4,9, Ngian Chye Tan3,4,9, N Gopalakrishna Iyer3,4,9 and Hiang Khoon Tan3,4,9
1Physiotherapy Department, Singapore General Hospital, Singapore
2Ministry of Health Holdings, Singapore
3Head and Neck Surgery Department, Division of Surgery and Surgical Oncology, Singapore General Hospital, National Cancer Centre, Singapore
4SingHealth Duke-NUS Head & Neck Centre, Singapore
5Centre for Quantitative Medicine, Duke-NUS Medical School, Singapore
6Curtin School of Allied Health, Curtin University, Australia
7Curtin enAble Institute, Curtin University, Australia
8Radiography Department, Division of Radiological Sciences, Singapore General Hospital, Singapore
9SingHealth Duke-NUS Global Health Institute, Singapore
*Address for Correspondence: Celia Ia Choo Tan, PhD, Senior Principal PT, Physiotherapy Department, Singapore General Hospital, SingHealth Tower, 10 Hospital Boulevard, Singapore 168582, Singapore, Email: celia.tan.i.c@singhealth.com.sg
An established side-effect of neck dissection (ND) for head and neck (HNC) tumour management includes shoulder dysfunction (SD), which can impact quality of life (QOL). Shoulder strength and range of movement (ROM) are key parameters to be monitored in SD. However, such evaluations are not routinely conducted in the clinical setting. The aim of this study was to evaluate objectively the impact of ND on shoulder functions.
Methods: This is a pilot exploratory study in a tertiary cancer centre. Five participants with unilateral ND and advanced HNC, completed the study. Outcome measures consisted of self-reported QOL questionnaires, C2–T1 dermatomes and shoulder ROM and strength testing. Data was collected at baseline, 1.5-months after surgery and 6-months after diagnosis (after adjuvant treatment completion).
Results: Most outcome measures on the surgically affected side were negatively impacted post-operatively, with varied recovery seen at follow-up. Sensory loss was noted at C3–4 dermatome levels. Shoulder ROM and strength was reduced on the surficial side for all participants, with some recovery after six months except for two participants.
Conclusion: Results of SD after ND are diverse and unique to each patient. Findings from this pilot study indicate that regular rehabilitation/exercise may facilitate recovery of shoulder function post HNC surgery. However, customised rehabilitation may yield better outcomes. Future studies with a larger sample are indicated to validate the findings of this study.
Treatment for advanced head and neck cancers (HNCs) is usually multimodal comprising of surgery followed by either adjuvant radiation or chemo-radiation therapy (RT/CRT) [1]. Neck dissection (ND) is an effective surgical procedure performed for the removal of cervical metastasis in HNC treatment. One of the common side-effects of ND is some degree of shoulder dysfunction (SD)[2-5]. This can lead to a reduction in quality of life (QOL) in HNC patients after ND [2,3].
Advances in HNC treatment have evolved to improve survival and QOL outcomes [1]. Studies validate that despite nerve preserving NDs, SD can still occur in 20% to 60% of these patients [6,7]. There is evidence that exercise has a positive impact on QOL among individuals with cancer [8]. Yet less than half of HNC survivors engage in regular exercise, due to fatigue, shoulder pain and disability [9,10].
Specific measurements of shoulder function, both sub-jective and objective, are not routinely employed clinically to report SD. This knowledge has the potential to facilitate prognostication, risk-stratification and customised rehabili-tation programs. However, clinicians and cancer survivors are more concerned about surveillance of cancer recurrence such that rehabilitation does not always receive the attention and time it deserves and at best, it may be reduced to a one-time assessment [11].
Currently, the impact of SD among HNC patients after ND and adjuvant treatment in Singapore is largely unknown. The primary aim of this pilot, exploratory study was to evaluate shoulder functions after surgery and adjuvant treatment, to understand the extent of SD from each of these treatments.
Study design
This was a pilot, exploratory prospective cohort study conducted from October 2017 to March 2019. Patients were recruited and consent taken at an out-patient dedicated HNC clinic at a tertiary care comprehensive cancer centre, after verification of data on electronic medical records (EMR). This study received ethical approval from Institutional Review Board committee (CIRB no. 2016-2578).
Inclusion criteria: HNC patients aged between 20 to 70 years, who were (1) diagnosed with histologically proven advanced cancers of the upper aerodigestive tract (i.e., oral cavity, pharynx, and larynx) and salivary glands; (2) awaiting surgery and likely to undergo adjuvant treatment.
Exclusion criteria: HNC patients who were (1) diagnosed with head and neck cutaneous malignancies, thyroid cancer, or with distant metastases; (2) unfit for surgery on account of medical co-morbidities; (3) unfit for rehabilitation due to the presence of other illnesses/injury (i.e., history of shoulder surgery, stroke, ischaemic heart disease).
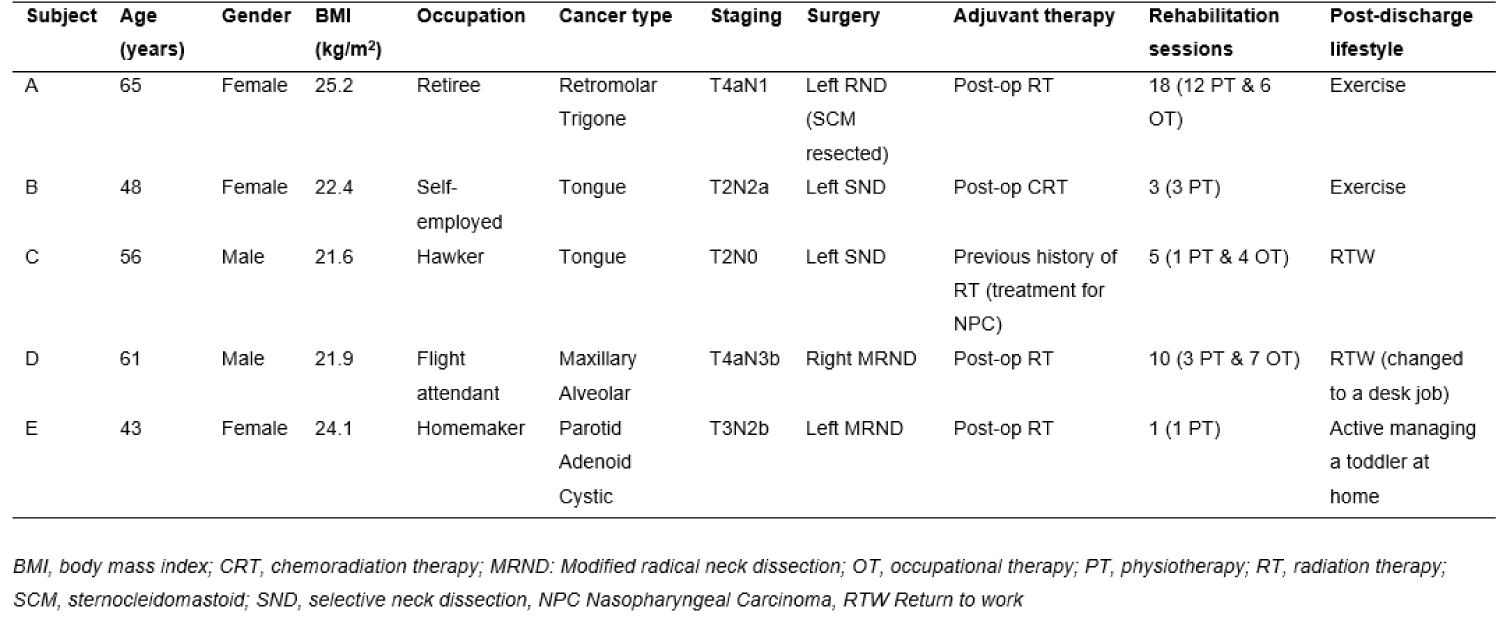
Ten participants were recruited under a broader study titled “The impact of multimodality treatment on physical functions and QOL in patients with HNC: A prospective cohort study”. Five participants had unilateral ND and the other five had bilateral ND. In this paper, we focused on the five patients with unilateral ND. Each participant had an affected side (ipsilateral to surgery), and unaffected side (contralateral to surgery) that served as control.
1. Clinical and patient characteristics sociodemographic data: Age, gender and body mass index (BMI).
2. Clinical data: Cancer site, stage, treatment modality and rehabilitation sessions.
Outcome, easures:
3. QOL Questionnaires: European Organization for Research and Treatment of Cancer - Quality of Life Questionnaire (EORTC QLQ-C30) and Shoulder Pain and Disability Index (SPADI).
4. Sensory mapping of neck and shoulder region.
5. Shoulder range of motion (ROM) and strength.
Testing for the participants was conducted at the Movement Science Laboratory at the Tertiary Care facility, across three visits. Baseline testing was conducted at visit 1, prior to surgery (termed “baseline”). Post-operative testing was conducted during visit 2, scheduled one and a half months after surgery before radiation therapy commenced (termed post-op). A final follow-up test was conducted during visit 3 at six months, after completion of radiation therapy (termed “follow-up”).
Subjective outcome measures
QOL questionnaires: All participants completed the EORTC QLQ-C30 and SPADI. The EORTC QLQ-C30 incorporates a global health status/QOL scale, where higher scores indicate improved QOL. In SPADI, lower scores in pain and disability scales correspond to improved shoulder function.
Sensation mapping: Light touch was applied around the neck and shoulder region at dermatome distribution levels of C2 to T1 on both affected and unaffected sides, modified from the American Spinal Injury Association’s Assessment Scoresheet [12] . The participants were asked to report any sensory difference between tested area versus anterior thigh, which served as control. Results were recorded as absent (0), diminished (1) or normal (2); and this testing took about 5 to 10 minutes to complete for each participant.
Objective outcome measures
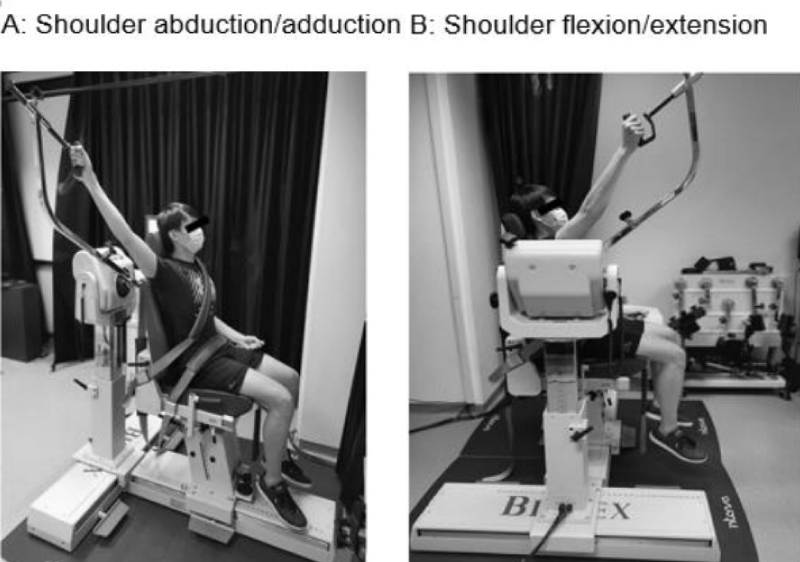
Shoulder ROM: Participants were seated and strapped to a Biodex dynamometer (Biodex Multi-Joint System PRO #850-000, Biodex Medical Systems, New York USA) to ensure consistent posture during measurements. Active ROM was performed with the dynamometer, while passive ROM was done with a hand-held 360-degree goniometer (Baseline #12-1000, New York USA) by an experienced physiotherapist, in the same resting position. Each participant received standardised instructions, and an average of two measure-ments in shoulder abduction and flexion was taken (Figure 1).
Figure 1: Set up for active ROM and strength assessments in (A) shoulder abduction and (B) shoulder flexion.
Shoulder strength: The dynamometer was also used for strength assessments in shoulder abduction and flexion. Isometric strength (maximal voluntary contraction) was conducted at 45° angle, while isokinetic strength (peak torque) was performed from 45° to 120° angle at a default speed of 120°/s. The higher of two measurements was recorded. Data collection was terminated if the participant could not perform the assessment due to pain or any physical impairment. Testing for ROM and strength took about 30 minutes to complete for each participant (Figure 1).
Statistical analysis
All analysed variables were reported at group and individual patient level. Assessment of dermatome at 8 different positions were reported as box plots. Shoulder ROM and isokinetic/isometric strength measurements at baseline (0 month), post-operatively (1.5 months) and follow-up (6 months) were reported as box plots. The difference in distribution of shoulder ROM and isokinetic/isometric strength measurements over time was tested using Mann–Whitney U test. All analyses were performed using the SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Demographics
All five participants were diagnosed with advanced HNC (four with oral cavity cancer and one with salivary gland cancer). The group comprised of 40% male (n = 2) and 60% females (n = 3), with a median age of 56 years. All participants underwent wide-resection of the primary tumour and unilateral functional ND with preservation of SAN: two had modified radical ND (MRND) levels 1-5, two had selective-ND (SND) levels 1-3, and one had MRND with resection of the sternocleidomastoid (SCM) muscle.
Four of them received adjuvant RT treatment, except for participant C who had N0 neck where post-operation RT was not indicated. In addition, participant C had previous history of RT for treatment of nasopharyngeal carcinoma (NPC) 11 years ago, and participant D had regional recurrence of cancer after surgery.
Post-operative rehabilitation
All participants received in-patient rehabilitation, ranging from one to 18 sessions, by the physiotherapist, to assess and support recovery. The sessions were arranged on per needs basis, hence the variation in treatment numbers provided. Participant A who underwent RND, received the most rehabilitation sessions (18 sessions) (Table 1).
Table 1: Cohort Characteristics.
After discharge, all participants remained active either through weekly exercise (Participants A and B) or returning to work (Participants C and D). Participant D changed the work to a more sedentary, administrative position. Participant E was engaged in childcare and domestic tasks.
Subjective outcome measures
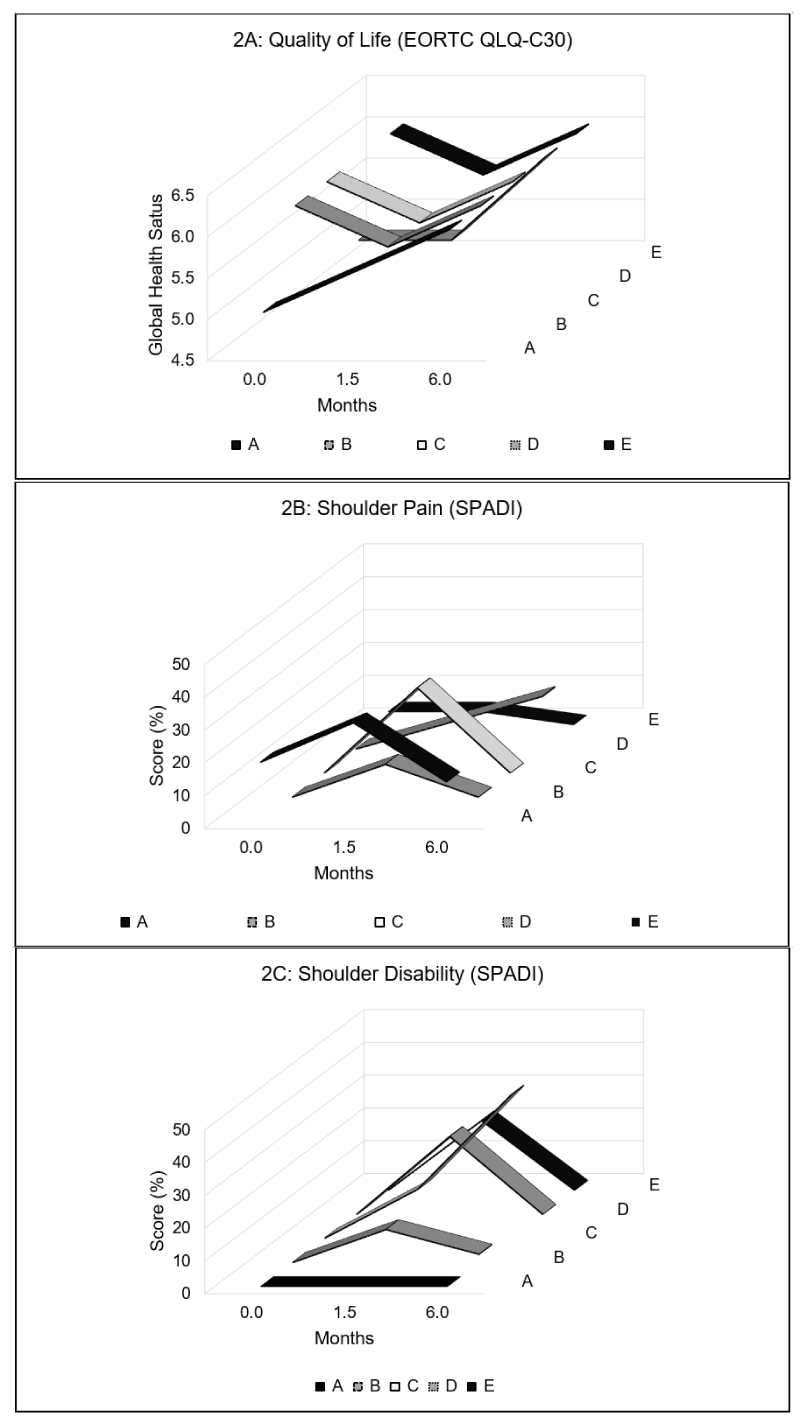
Quality of life: Figure 2A, QOL was negatively impacted post-operatively for three participants (B, C and E). During follow-up, QOL had improved back to baseline, with two participants (A and B) exceeding their baseline QOL score.
Shoulder dysfunction: Figures 2B,C, All participants presented with increased SPADI post-operatively on the affected side. These scores, however, improved in most participants at follow-up, except for two participants (C and D) who continued to have residual impairments. Participant C reported shoulder disability at follow-up with no pain (Figure 2C) while Participant D complained of pain without shoulder disability (Figure 2B).
Figure 2: Questionnaire scores for (A) quality of life, (B) shoulder pain and (C) shoulder disability at baseline (0 month), post-op (1.5 months) and follow-up (6 months).
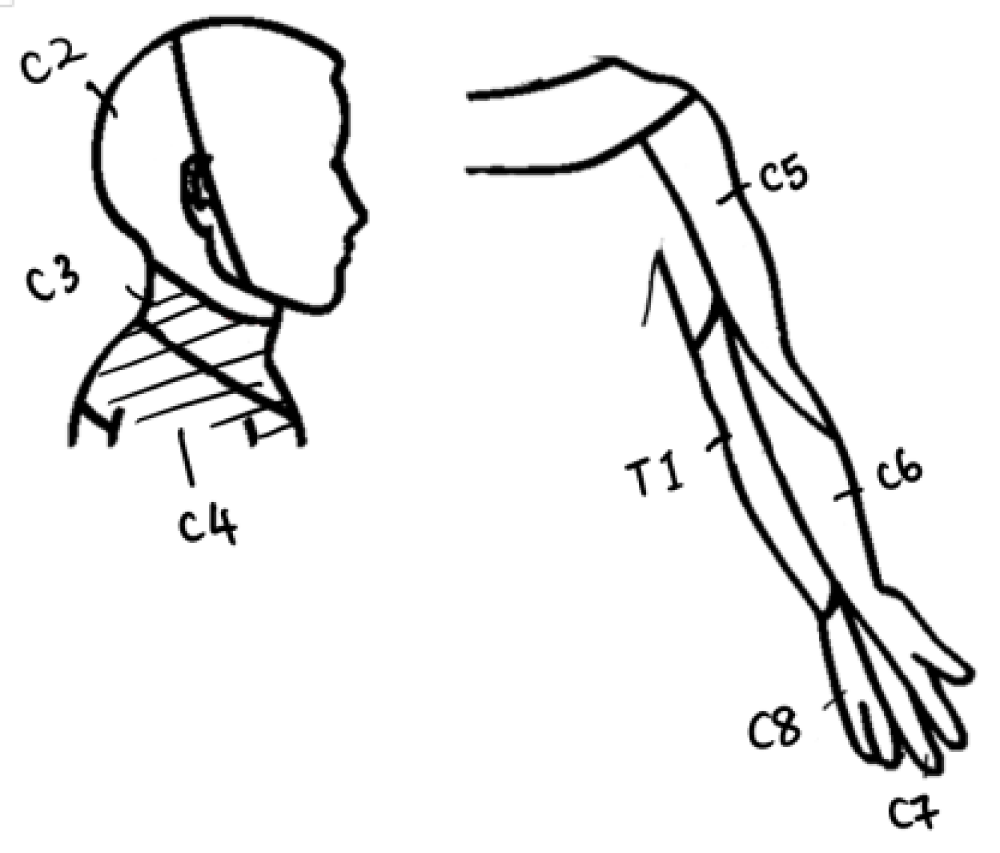
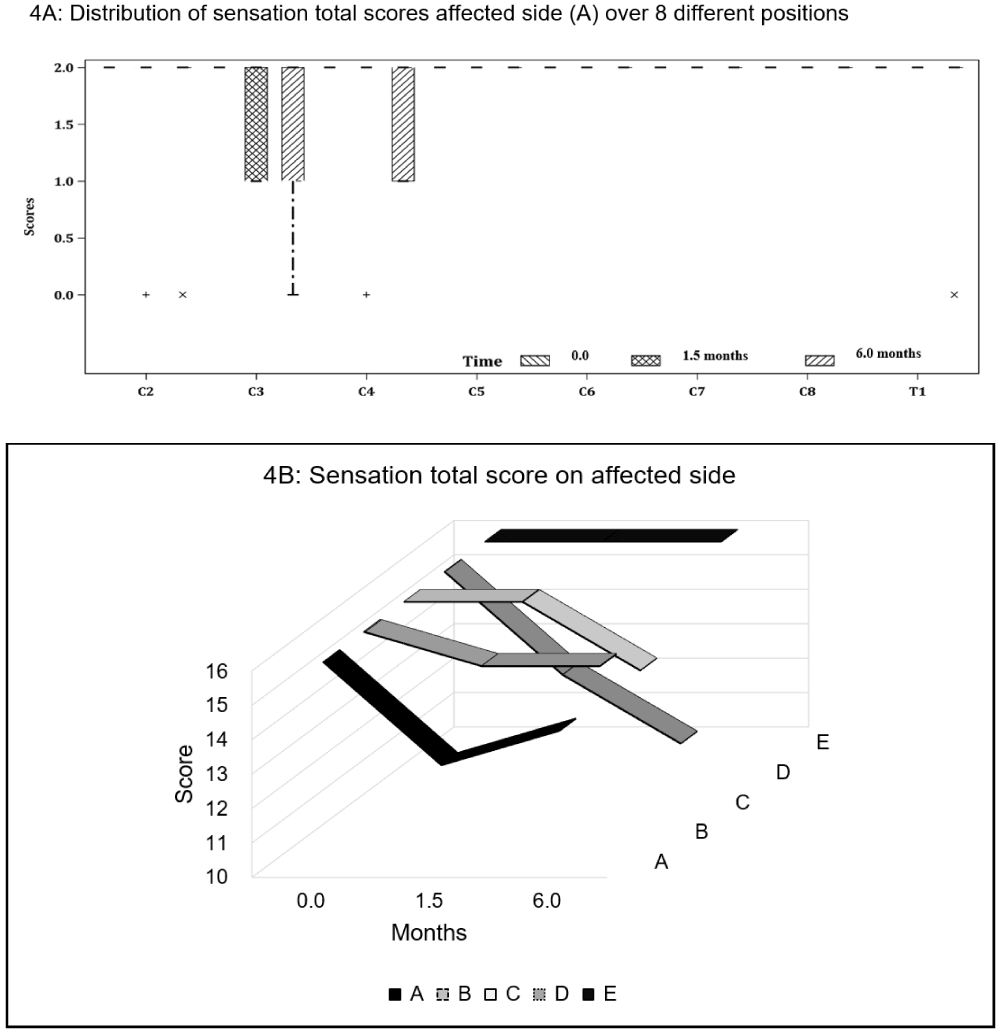
Neck sensation: Four participants exhibited sensory deficits post-operatively, with sensation being largely affected at C3–4 dermatome levels on the affected side. At follow-up, Participant A managed to recover partially her sensation loss at C4. Participant B continued to have diminished sensation at C3, whereas Participant C reported onset of absent sensation at T1. Participant D showed the most sensory deficits from post-operative period to follow-up, with worsening loss at C2 (0,0), C3 (1,0) and C4 (2,1) (Figures 3 and 4.)
Figure 3: Cervical spinal nerves dermatome distribution of the neck and upper limb. C3–4 (as shaded) are the most affected areas post-operatively in three of the five participants.
Figure 4: Sensation scores on the affected side for (A) all participants and (B) individual participants at baseline (0 month), post-op (1.5 months) and follow-up (6 months).
Objective outcome measures
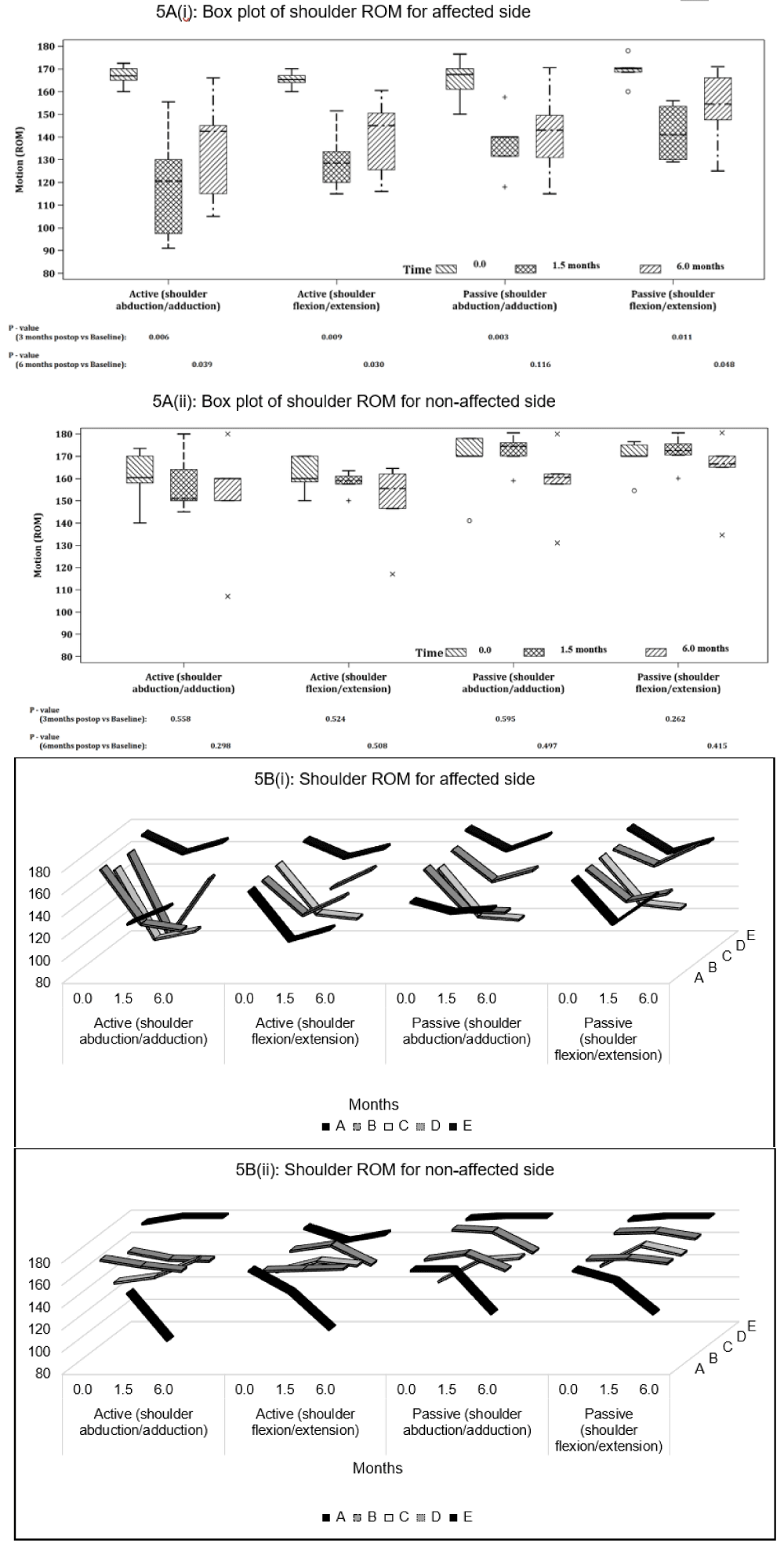
Shoulder ROM: Overall, active and passive flexion and abduction ROM measurements on the affected shoulder had significant reduction (p < 0.05) post-operatively, with active ROM declining more than passive ROM. Abduction ROM, in comparison to flexion ROM, was more affected and reduced although this was not statistically significant. At follow-up, only passive abduction ROM recovered close to baseline (p > 0.05) (Figures 5A,B).
Figure 5: Shoulder ROM (°) on the affected and unaffected side for active and passive abduction and flexion for (A) all participants and (B) individual participants at baseline (0 month), post-op (1.5 months) and follow-up (6 months). P - values are based on Mann-Whitney U-Test.
Participants B and C had reduced recovery in ROM on the affected arm as compared to the other participants (Figure 5B (i)). All participants did not have significant change in ROM of the unaffected shoulder throughout the study period (Figure 5B (ii)), except for Participant A. Participant A appeared to have a drop in both active and passive shoulder ROM for both flexion and abduction, as well as difficulty with testing even at baseline on the unaffected shoulder compared to the affected shoulder.
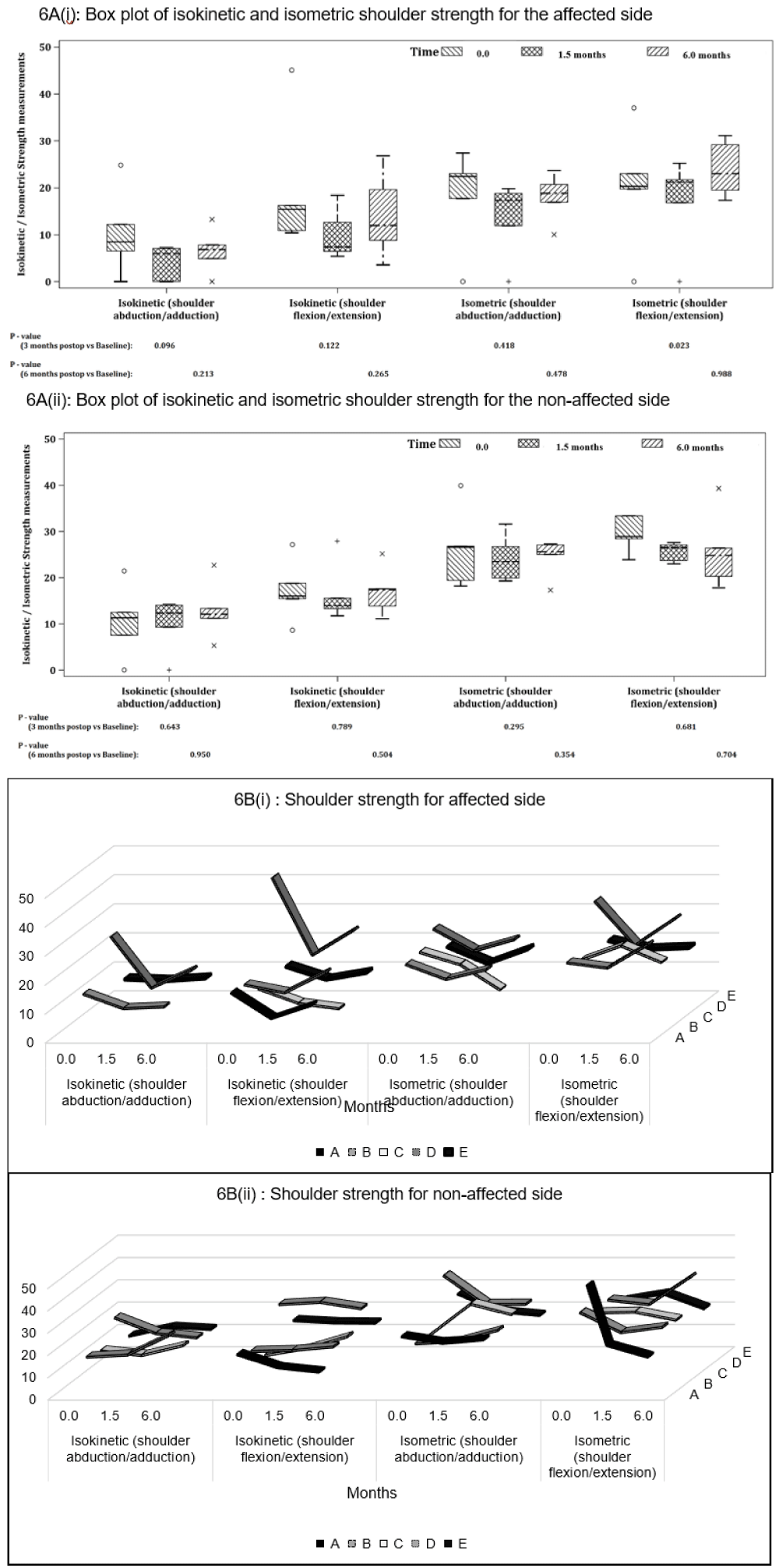
Shoulder strength: Overall, all strength measurements on the affected shoulder showed a decline at post-op and follow-up, however these changes were not statistically significant except for isometric flexion changes (p = 0.023) from baseline (Figure 6A(i)).
Overall isokinetic strength reductions were more apparent compared with isometric, and abduction strength was also lower than flexion values. At follow-up, the strength changes were more varied among the participants, hence the results were not statistically significant.
Participant A was not evaluated due to pain during assessment, hence there are missing data for isometric and isokinetic abduction strength (Figure 6B(i)). Participants B and E managed to recover the isometric and isokinetic strength back to baseline levels; but Participants C and D saw further deteriorations at follow up. The unaffected side showed no significant change in shoulder strength during the study period (Figures 6A(ii) and 6B(ii)).
Figure 6: houlder strength (N/m2) on the affected and unaffected side for isokinetic and isometric abduction and flexion for (A) all participants and (B) individual participants at baseline (0 month), post-op (1.5 months) and follow-up (6 months). P - values are based on Mann-Whitney U-Test.
In this study, all participants presented with below baseline scores in the subjective and objective outcome measures post-operatively. At follow-up, the subjective variables like QOL and SPADI improved back to baseline scores, but not so for the shoulder strength and ROM measurements, which continued to be lower compared to the baseline and the unaffected shoulder. Due to the small sample size, statistical significance could not be tested for all changes, hence clinical observations will be discussed for each subject instead.
Studies show overwhelming evidence for the benefits of exercise as adjunct care to reduce physical impairment for cancer patients [8-11] . In addition, rehabilitation is reported to play an important role in treating the “shoulder syndrome” by preventing adhesive capsulitis and reducing myofascial pain in the shoulder girdle [10]. In this study, we noted that rehabilitation for the shoulder post operatively, was not provided routinely but on a needs basis, which may account for the decrease in the shoulder ROM and strength in the affected shoulder noted post operatively. Fortunately, some of the participants (Participant A and B) continued to exercise on their own upon discharge, hence the varying levels of shoulder recovery noted among the five participants.
It was noted in Participant A, who initially reported high levels of shoulder pain which impeded her strength assessments post operatively as well as at baseline, hence the missing data (Figure 6B). Following a rehabilitation programme (12 PT and 6 OT sessions), she reported a reduction in pain to below baseline level and managed to regain strength at follow-up.
Similar shoulder improvement results were noted for Participant B who continued to exercise on his own, and Participant E who was engaged actively in childcare and domestic work, requiring use of the upper limbs. As such, it may be imperative that all HNC patients receive a prescribed dose of rehabilitation to reduce pain and SD. Future work in this area should investigate the optimal dose of exercise to encourage recovery.
On the other hand, Participant C, who received previous RT (history of NPC), reported increased shoulder disability throughout the study period. Although he returned to active work as a hawker after discharge, it did not seem to contribute to shoulder recovery in terms of strength and especially ROM. A possible reason for this participant’s poor post-operative recovery may be explained by existing soft tissue changes in the neck and shoulder region from previous RT and possibly radiation-induced fibrosis [13]; which may have already compromised musculoskeletal function before current treatment. Participant C started with both shoulder muscular weakness and limited passive ROM values below 130° at baseline, suggesting that ADLs may have already been compromised prior to the study. Oosterwijk, et al. [14] showed that in healthy individuals, shoulder flexion and abduction ROM of 130° was necessary to perform activities of daily living (ADLs).
Our study noted that participants were able to preserve isometric strength better than isokinetic strength in the affected shoulder after ND. Clinically, isometric exercises are usually conducted in the early phase of rehabilitation, especially where ROM is limited due to pain [15], and this may, in part explain our results. Dynamic or isokinetic shoulder exercises are more challenging to perform compared to isometric exercises as it requires an individual to generate shoulder force throughout the functional range of the joint [15]. However, the advantage of isokinetic exercise allows for improvements in more explosive muscular power type activities, akin to daily functional movements [15]. Hence, rehabilitation should also focus on isokinetic shoulder exercises for return to routine shoulder activities such as reaching, throwing, driving etc.
Loss of touch sensation was most affected at C3–4 dermatomes levels (Participants A, B and D). Saffold, et al. [16] Van Wilgen, et al. [17] and Garzaro, et al. [18], also showed that this region of the neck remained frequently affected, regardless of whether cervical plexus was sacrificed or spared during ND. Although damage to the cervical plexus reduces sensorial perception, it was not known to have any influence on shoulder pain that developed following ND [19] , as observed in our current study too.
Limitations
Inherent to a pilot exploratory study is selection bias. In addition, a small sample size necessitated case-series analysis. Further, the participants presented as a heterogenous group with differing factors such as cancer sites, stage, differing types of NDs, and frequency as well as type of rehabilitation. This made it challenging to analyse the results as a cohort and as such attribute a universal recommendation of rehabilitation for such cohorts.
In this study of five HNC participants, both subjective and objective measures of function were negatively affected at post-operative testing. There was improvement in shoulder ROM and strength in participants who undertook some form of exercise or rehabilitation. The results of this study forms the basis for future research for developing customised interventional strategies to reduce SD and improve QOL.
The authors would like to thank the doctors, physiotherapists and nursing team from National Cancer Centre and Singapore General Hospital for their support of the study.
- Robbins KT, Clayman G, Levine PA, Medina J, Sessions R, Shaha A, Som P, Wolf GT; American Head and Neck Society; American Academy of Otolaryngology--Head and Neck Surgery. Neck dissection classification update: revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology-Head and Neck Surgery. Arch Otolaryngol Head Neck Surg. 2002 Jul;128(7):751-8. doi: 10.1001/archotol.128.7.751. PMID: 12117328.
- Stuiver MM, van Wilgen CP, de Boer EM, de Goede CJ, Koolstra M, van Opzeeland A, Venema P, Sterken MW, Vincent A, Dijkstra PU. Impact of shoulder complaints after neck dissection on shoulder disability and quality of life. Otolaryngol Head Neck Surg. 2008 Jul;139(1):32-9. doi: 10.1016/j.otohns.2008.03.019. PMID: 18585558.
- Eickmeyer SM, Walczak CK, Myers KB, Lindstrom DR, Layde P, Campbell BH. Quality of life, shoulder range of motion, and spinal accessory nerve status in 5-year survivors of head and neck cancer. PM R. 2014 Dec;6(12):1073-80. doi: 10.1016/j.pmrj.2014.05.015. Epub 2014 May 28. PMID: 24880060; PMCID: PMC4247358.
- Ghiam MK, Mannion K, Dietrich MS, Stevens KL, Gilbert J, Murphy BA. Assessment of musculoskeletal impairment in head and neck cancer patients. Support Care Cancer. 2017 Jul;25(7):2085-2092. doi: 10.1007/s00520-017-3603-1. Epub 2017 Feb 13. PMID: 28191589.
- Gane EM, McPhail SM, Hatton AL, Panizza BJ, O'Leary SP. Neck and Shoulder Motor Function following Neck Dissection: A Comparison with Healthy Control Subjects. Otolaryngol Head Neck Surg. 2019 Jun;160(6):1009-1018. doi: 10.1177/0194599818821885. Epub 2019 Jan 22. PMID: 30665326.
- Gane EM, Michaleff ZA, Cottrell MA, McPhail SM, Hatton AL, Panizza BJ, O'Leary SP. Prevalence, incidence, and risk factors for shoulder and neck dysfunction after neck dissection: A systematic review. Eur J Surg Oncol. 2017 Jul;43(7):1199-1218. doi: 10.1016/j.ejso.2016.10.026. Epub 2016 Nov 17. PMID: 27956321.
- Almeida KAM, Rocha AP, Carvas N, Pinto ACPN. Rehabilitation Interventions for Shoulder Dysfunction in Patients With Head and Neck Cancer: Systematic Review and Meta-Analysis. Phys Ther. 2020 Oct 30;100(11):1997-2008. doi: 10.1093/ptj/pzaa147. PMID: 32750136.
- Gerritsen JK, Vincent AJ. Exercise improves quality of life in patients with cancer: a systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2016 Jul;50(13):796-803. doi: 10.1136/bjsports-2015-094787. Epub 2015 Dec 30. PMID: 26719503.
- Rogers LQ, Courneya KS, Robbins KT, Malone J, Seiz A, Koch L, Rao K, Nagarkar M. Physical activity and quality of life in head and neck cancer survivors. Support Care Cancer. 2006 Oct;14(10):1012-9. doi: 10.1007/s00520-006-0044-7. Epub 2006 Mar 15. PMID: 16538497.
- Sammut L, Ward M, Patel N. Physical activity and quality of life in head and neck cancer survivors: a literature review. Int J Sports Med. 2014 Aug;35(9):794-9. doi: 10.1055/s-0033-1363984. Epub 2014 Feb 19. PMID: 24554555.
- Guru K, Manoor UK, Supe SS. A comprehensive review of head and neck cancer rehabilitation: physical therapy perspectives. Indian J Palliat Care. 2012 May;18(2):87-97. doi: 10.4103/0973-1075.100820. PMID: 23093823; PMCID: PMC3477371.
- American Spinal Injury Association (ASIA). International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) Worksheet. Retrieved from https://asia-spinalinjury.org/international-standards-neurological-classification-sci-isncsci-worksheet/ (accessed 25/05/2021).
- Hunter KU, Jolly S. Clinical review of physical activity and functional considerations in head and neck cancer patients. Support Care Cancer. 2013 May;21(5):1475-9. doi: 10.1007/s00520-013-1736-4. Epub 2013 Feb 17. PMID: 23417564.
- Oosterwijk AM, Nieuwenhuis MK, van der Schans CP, Mouton LJ. Shoulder and elbow range of motion for the performance of activities of daily living: A systematic review. Physiother Theory Pract. 2018 Jul;34(7):505-528. doi: 10.1080/09593985.2017.1422206. Epub 2018 Jan 29. PMID: 29377745.
- Lee SEK, Lira CAB, Nouailhetas VLA, Vancini RL, Andrade MS. Do isometric, isotonic and/or isokinetic strength trainings produce different strength outcomes? J Bodyw Mov Ther. 2018 Apr;22(2):430-437. doi: 10.1016/j.jbmt.2017.08.001. Epub 2017 Aug 19. PMID: 29861246.
- Saffold SH, Wax MK, Nguyen A, Caro JE, Andersen PE, Everts EC, Cohen JI. Sensory changes associated with selective neck dissection. Arch Otolaryngol Head Neck Surg. 2000 Mar;126(3):425-8. doi: 10.1001/archotol.126.3.425. PMID: 10722022.
- van Wilgen CP, Dijkstra PU, van der Laan BF, Plukker JT, Roodenburg JL. Morbidity of the neck after head and neck cancer therapy. Head Neck. 2004 Sep;26(9):785-91. doi: 10.1002/hed.20008. PMID: 15350024.
- Garzaro M, Riva G, Raimondo L, Aghemo L, Giordano C, Pecorari G. A study of neck and shoulder morbidity following neck dissection: The benefits of cervical plexus preservation. Ear Nose Throat J. 2015 Aug;94(8):330-44. PMID: 26322451.
- Dilber M, Kasapoglu F, Erisen L, Basut O, Tezel I. The relationship between shoulder pain and damage to the cervical plexus following neck dissection. Eur Arch Otorhinolaryngol. 2007 Nov;264(11):1333-8. doi: 10.1007/s00405-007-0357-2. Epub 2007 Jun 7. PMID: 17554547.