More Information
Submitted: August 16, 2022 | Approved: August 25, 2022 | Published: August 26, 2022
How to cite this article: Wei M, Yang R. Viscosity-sensitive mitochondrial fluorescent probes and their bio-applications. Ann Adv Chem. 2022; 6: 038-042.
DOI: 10.29328/journal.aac.1001029
Copyright License: © 2022 Wei M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Mitochondria; Viscosity; Fluorescent probe; Biological imaging
Viscosity-sensitive mitochondrial fluorescent probes and their bio-applications
Mengmeng Wei and Rui Yang*
School of Electronics & Information Engineering, Changshu Institute of Technology, Changshu, 215500, China
*Address for Correspondence: Rui Yang, School of Electronics & Information Engineering, Changshu Institute of Technology, Changshu, 215500, China, Email: yangruithrive@163.com; 2364691528@qq.com
As a vital index of the mitochondrial micro-environment, mitochondrial micro-viscosity plays a fundamental role in cell life activities. Normal mitochondrial viscosity is a necessary condition for the maintenance of normal life activities of mitochondria. Abnormal mitochondrial viscosity can lead to a series of mitochondria-related diseases. Therefore, it is essential to observe mitochondrial viscosity for physiological and pathological processes. Given the conventional viscosity measurement methods (viscometer, etc.) cannot monitor the changes in mitochondrial viscosity, the fluorescence method supplemented with the fluorescent probe is widely used to observe the changes in mitochondrial viscosity. In view of the booming development in this area, this review describes the applications of viscosity-responsive mitochondrial fluorescent probes in biological samples from the cellular and tissue levels. We hope that this review will deepen our understanding of mitochondrial viscosity and related fields, and promote the development of viscosity-sensitive mitochondrial probes and other organelle fluorescence probes.
As we all know, a relatively constant intracellular microenvironment is crucial for cells to maintain normal life activities. An abnormal intracellular microenvironment will interfere with cell life activities and cause a series of diseases such as hypertension and diabetes [1,2]. As an important class of intracellular microenvironment, viscosity is closely related to physiological and pathological processes such as signal transduction, metabolite diffusion, and biomolecular interaction, so it is crucial to detect viscous changes in living cells [3]. Mitochondria are a kind of important organelle in cells, and mitochondria are involved in a variety of cellular life processes, including ROS generation, ATP synthesis, apoptosis, and cell differentiation [4-6].
Mitochondrial function is closely related to mitochondrial viscosity and mitochondrial metabolism. Mitochondrial viscosity can regulate related biological processes and affect mitochondrial protein regulation, respiratory status, and signal transduction [7,8]. Abnormal changes in mitochondrial viscosity can damage cell function, accelerate aging, and lead to diabetes, Parkinson’s disease, Alzheimer’s disease and many other diseases [9-12]. Therefore, it is important to visualize changes in mitochondrial viscosity for the diagnosis of corresponding diseases, but it remains a great challenge.
For a uniform and stable environment, the viscosity can be measured with a viscometer (e.g., rotary viscometer, capillary viscometer, etc.). For specific microenvironments, especially organelle viscosity in cells, macroscopic testing tools such as viscometers do not work [13,14]. Magnetic resonance elastography (MRE) is a new imaging technology that can measure hardness and viscosity simultaneously. However, its imaging resolution is low and it cannot measure intracellular mitochondrial viscosity [15,16]. Fluorescence technology has the unique advantages of strong selectivity, simple operation, and little damage. Fluorescence microscopy can be used to visualize organelles of living cells [17-19] and the subcellular life activity in living cells can be revealed by measuring specific subcellular microenvironment information with fluorescent probes [20].
Based on this method, viscosity-sensitive fluorescent probes targeting mitochondria can be developed to observe changes in mitochondrial viscosity.
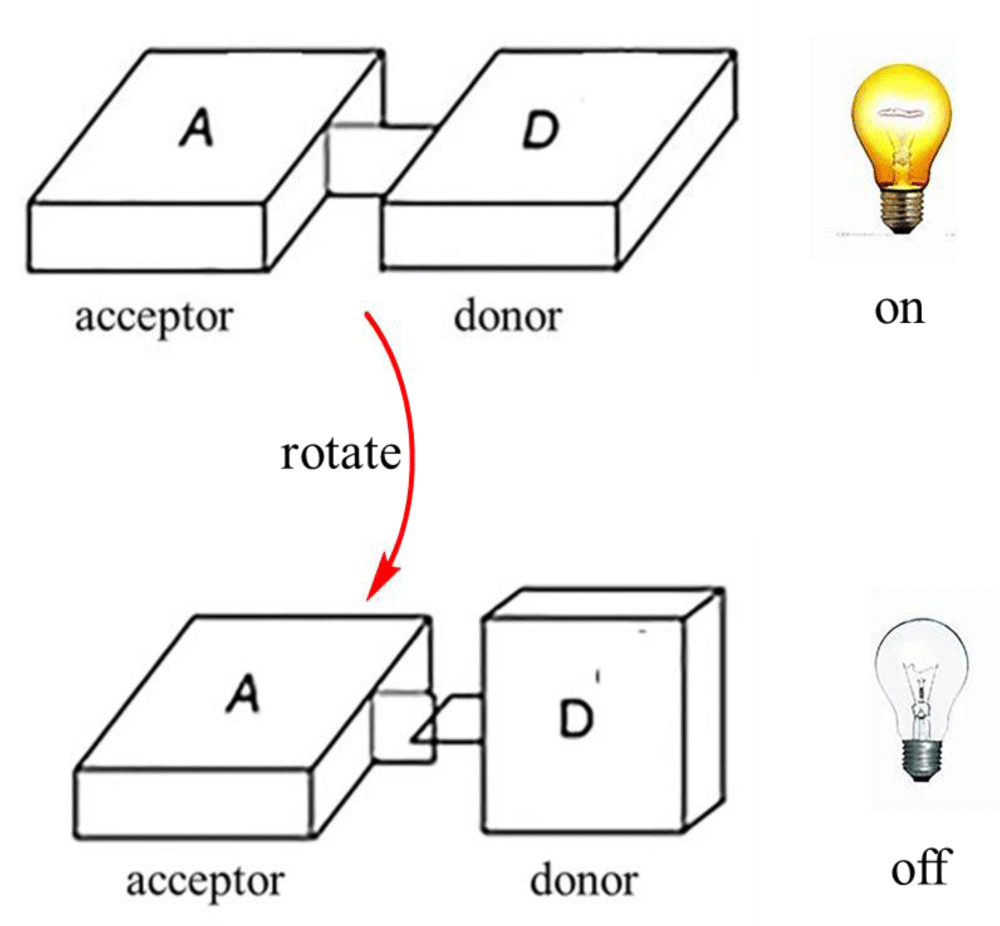
Viscosity-sensitive mitochondrial fluorescent probes have made significant progress in the past few years, and many fluorescent probes have been developed for measuring mitochondrial viscosity. Typically, such probes usually contain a fluorophore and a rotor. In general, the molecular rotor can rotate relative to the fluorophore in a medium with low viscosity, and its fluorescence intensity is weakened or its fluorescence lifetime is shortened. In contrast, in highly viscous media, molecular rotation is inhibited, and the fluorescence signal or fluorescence lifetime is significantly enhanced. Due to the high mitochondrial membrane potential (MMP), the probes with positive ion salts were designed to target mitochondria. So, the rotors with positive ion salts are suitable for visualizing mitochondria. The overall design concept and the sensing mechanism of the probe for mitochondrial viscosity are shown in Figure 1. In this review, we mainly introduce the application of viscosity-sensitive mitochondrial fluorescent probes in imaging cells and biological tissue samples in recent years. We expect that this review will attract a broad audience from sensors and microenvironments, and promote the development of detection, optical reagent imaging, biology, and medical science.
Figure 1: Viscosity identification mechanism of molecular probes.
Application of viscosity-sensitive mitochondrial probe
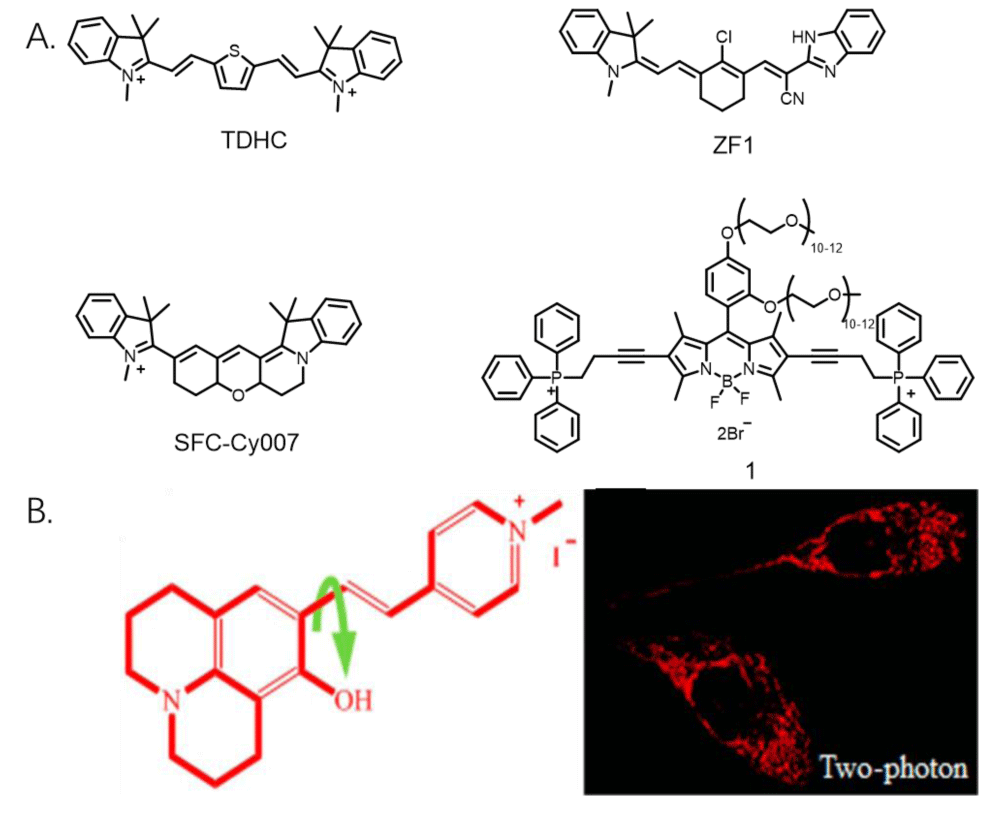
Measurement of mitochondrial viscosity in living cells: In general, mitochondrial membrane viscosity is high in living cells and can be measured by probes targeting the mitochondrial membrane, which is the most common way to measure mitochondrial viscosity. Baek, et al. developed sensitive fluorescent dyes (TDHC, Figure 2A) which can real-time monitor the viscosity of the motor neuron mitochondria. The dye has good stability, and it can be used for high-fidelity imaging of mitochondrial transport. So, the process of tubular mitochondria’ rapid transport along the dendrites and axons in primary cortical neurons was clearly visualized [2]. Park, et al. developed an asymmetric far-infrared mitochondrial fluorescence probe (SFC-CY007, Figure 2A) [21], which can observe changes in mitochondrial viscosity in cells. In addition, SFC-CY007 has high brightness and photostability, which is expected to be used to observe mitochondria-related diseases. Zhang, et al. developed a novel viscosity-sensitive fluorescent probe with a large Stokes shift (ZF1, Figure 2A), which has a near-infrared fluorescence emission peak and a large Stokes shift, and it can be used to observe mitochondrial viscosity. Sui, et al. designed and developed a water-soluble fluorescent probe that can target mitochondria (probe 1, Figure 2A). The probe consists of three parts: BODIPY (fluorophores), TPP (mitochondrial targeting groups), and PEG (increased water solubility), and the probe has high water solubility, high fluorescence quantum yield, good photostability, low cytotoxicity, and excellent mitochondrial targeting properties [22]. Zhang, et al. developed a viscosity-sensitive mitochondrial probe (HJVPI, Figure 2B) that can be used to image mitochondria with high fidelity [23]. The number of such probes is relatively large, and they can visualize mitochondria viscosity in living cells. However, such probes cannot quantify mitochondrial viscosity, and the study of probes that enables quantifying mitochondrial viscosity is still in progress.
Figure 2: A. Several probe structures for measuring mitochondrial viscosity in living cells. B. Structural formula and cell imaging pictures of probe HJVPI.
Simultaneous measurement of mitochondrial viscosity and ions in living cells
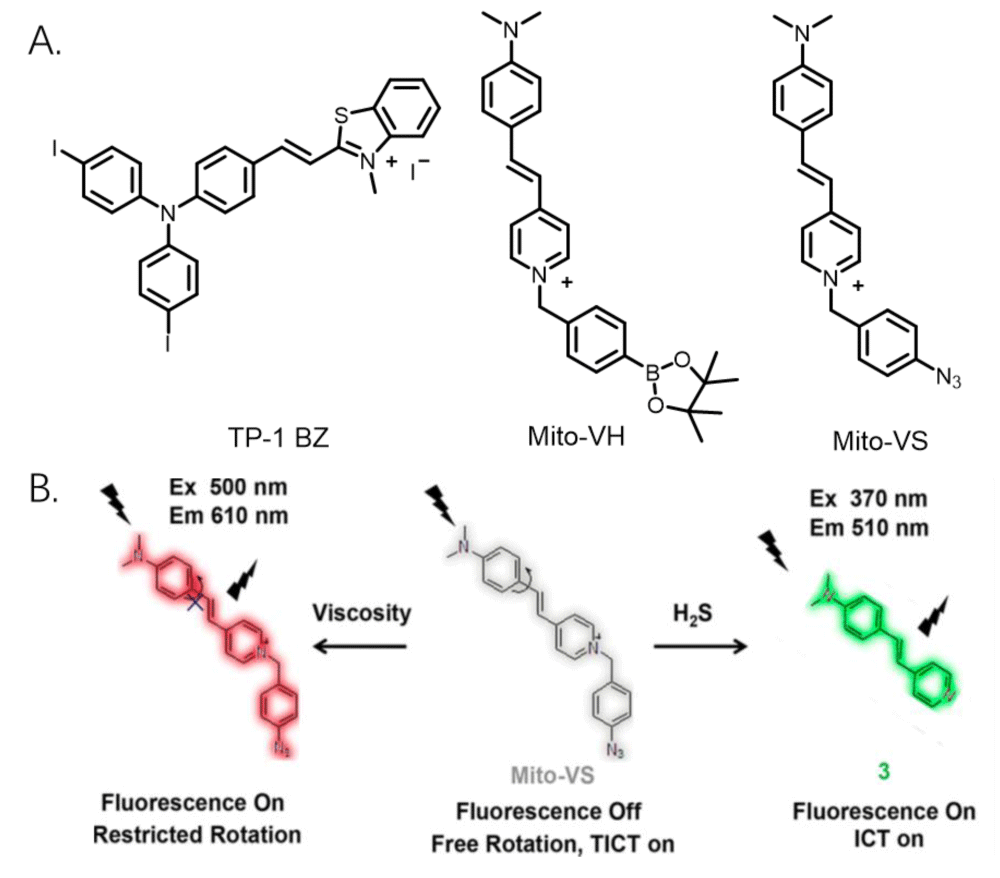
Mitochondria contain many ions and biological macromolecules, and these ions, biological macromolecules, and the microenvironment of mitochondria jointly affect the physiological function of mitochondria. Therefore, probes that can observe the ions and viscosity of mitochondria are helpful to monitor the physiological state of mitochondria. Sun, et al. developed a two-photon ratio fluorescent probe (TP-1BZ, Figure 3A) that can simultaneously visualize mitochondrial viscosity and ONOO-. The probe shows high targeting to mitochondria and high response to viscosity, and it can observe the changes in mitochondrial viscosity. In addition, the probe also shows the high response to ONOO-. Its detection limit is 12.1 nM, which further indicates its potential application in pathology [24]. Ren, et al. developed a fluorescent probe that could image mitochondrial viscosity and H2O2 in living cells with different fluorescence signals (MitO-VH, Figure 3A). When the probe responded to the viscosity, it would emit a fluorescence peak of 607 nm (red fluorescence), and when the probe responded to H2O2, it would emit a fluorescence peak of 510 nm (green fluorescence). Therefore, the probe can observe viscosity and H2S in mitochondria with two fluorescence colors [25]. Li, et al. developed a dual-response fluorescent probe (MitO-VS, Figure 3A, 3B) for viscosity and hydrogen sulfide detection, which can observe changes in mitochondrial viscosity in the red channel and image H2S in the green channel to achieve dual-channel imaging of viscosity and H2S [26]. This probe can observe the ions and viscosity of mitochondria, which is useful for biologists. However, these kinds of probes are much less, and it is hard to design and synthesize the probes. The probes able to image mitochondrial viscosity and other ions are still in progress.
Figure 3: A. Several fluorescent probe structures can identify mitochondrial viscosity and ions in living cells. B. Recognition mechanism of probe MitO-VS.
Observation of mitochondrial viscosity in tissues
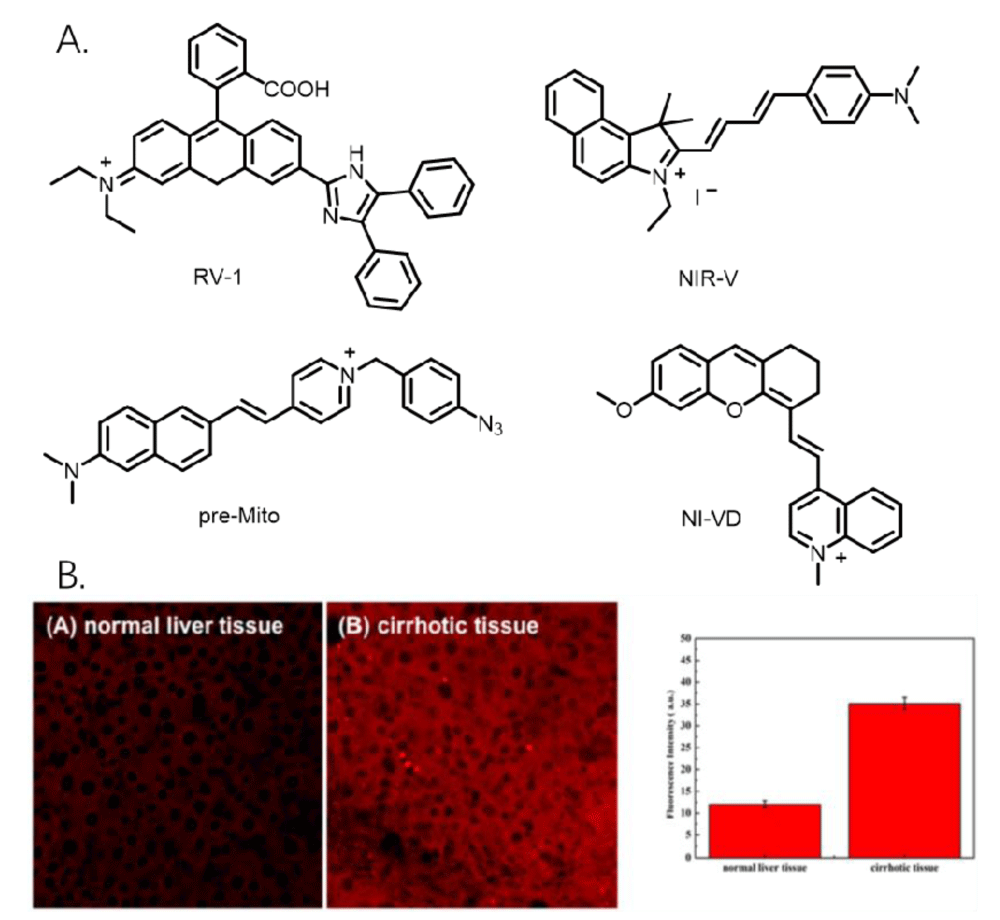
Compared with cells cultured in vitro, it is difficult to image organelles in tissues. Mainly because biological tissues contain a variety of ions and biological macromolecules, and their composition is relatively complex. In addition, compared with visualizing tissues, many cellular states and essential functions presented in tissues are often missed when imaging cells cultured in vitro, so imaging intracellular organelles in tissues are more exquisite and reliable for biologists, which creates difficulties in imaging organelles in tissues [27]. Guo, et al. developed a novel mitochondria-targeted rhodamine analog (RV-1, Figure 4A), which is sensitive to mitochondrial viscosity and can detect viscosity changes in living cells, zebrafish, and live mice [28]. Chen, et al. developed a viscosity-sensitive near-infrared mitochondria fluorescent probe (NIR-V, Figure 4A) that can be used to image a diabetic mouse model [29]. Fang, et al. developed a dual-channel fluorescent probe for in-situ imaging mitochondrial H2S/viscosity (pre-MITO, Figure 4A), which can distinguish mitochondrial H2S/ viscosity in the brain of a drosophila model of Parkinson’s disease, and it exhibits low H2S and high viscosity compared with normal flies [30]. Dai, et al. developed a viscosity-sensitive near-infrared fluorescent probe (NI-VD, Figure 4A) that can observe viscosity changes in mitochondria in cells and organisms. Importantly, it can distinguish normal kidneys from diabetic kidneys by detecting changes in cell viscosity [31]. Zhang, et al. developed a viscosity-responsive probe that can distinguish normal and cirrhotic mouse liver tissues (Figure 4B) [32]. The probe has weak fluorescence in normal liver tissues and strong fluorescence intensity in cirrhotic tissues, thus it was applied to distinguish between normal and cirrhotic mouse tissues. The probes have high permeability to tissues, and they can visualize the viscosity changes in mitochondria. However, these probes are not able to qualify the viscosity of mitochondria in tissues. Moreover, due to the relatively complex environment in tissues, there are few studies on this kind of probe, and we hope to make further progress on this kind of probe.
Figure 4: A. Several fluorescent probe structures for measuring mitochondrial viscosity in tissues. B. Tissue image of the probe.
Observing mitochondrial viscosity in cells and tissues is of great interest to physiologists and pathologists, and the research in this area has also attracted the interest of experts in the field of fluorescent probes. Currently, viscosity-sensitive fluorescent probes have good targeting ability to mitochondria, high fluorescence intensity, and quantum yield, and they can observe the changes of viscosity in cells and tissues. Although the current mitochondrial-sensitive fluorescent probe can quantify the viscosity in solvents, it still cannot achieve the quantitative detection of viscosity in cells and tissues, and the related research is still in progress. In addition, this paper will help readers understand the relationship between mitochondrial viscosity and health/disease, and we look forward to achieving exciting progress through the collaboration of experts from all fields. Therefore, we hope that this paper can promote the research progression of diseases related to mitochondrial viscosity.
The study was financially supported by Youth Foundation of Jiangsu Province (BK20220689), and the startup fund of the Changshu Institute of Technology (KYZ2021055Q, KYZ2021056Q).
- Lee SX, Lim HN, Ibrahim I, Jamil A, Pandikumar A, Huang NM. Horseradish peroxidase-labeled silver/reduced graphene oxide thin film-modified screen-printed electrode for detection of carcinoembryonic antigen. Biosens Bioelectron. 2017 Mar 15;89(Pt 1):673-680. doi: 10.1016/j.bios.2015.12.030. Epub 2015 Dec 15. PMID: 26718548.
- Baek Y, Park SJ, Zhou X, Kim G, Kim HM, Yoon J. A viscosity sensitive fluorescent dye for real-time monitoring of mitochondria transport in neurons. Biosens Bioelectron. 2016 Dec 15;86:885-891. doi: 10.1016/j.bios.2016.07.026. Epub 2016 Jul 9. PMID: 27494813.
- Zhang L, Guo R, Hu Q. A novel near-infrared probe with large Stokes shift for the detection of viscosity changes in living cells. Journal of Luminescence. 2021; 233: 117883.
- Yang R, He X, Niu G, Meng F, Lu Q, Liu Z, Yu X. A Single Fluorescent pH Probe for Simultaneous Two-Color Visualization of Nuclei and Mitochondria and Monitoring Cell Apoptosis. ACS Sens. 2021 Apr 23;6(4):1552-1559. doi: 10.1021/acssensors.0c02372. Epub 2021 Feb 3. PMID: 33533249.
- Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010 Sep;11(9):621-32. doi: 10.1038/nrm2952. Epub 2010 Aug 4. PMID: 20683470.
- Zhao RZ, Jiang S, Zhang L, Yu ZB. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int J Mol Med. 2019 Jul;44(1):3-15. doi: 10.3892/ijmm.2019.4188. Epub 2019 May 8. PMID: 31115493; PMCID: PMC6559295.
- Acin-Perez R, Enriquez JA. The function of the respiratory supercomplexes: the plasticity model. Biochimica et Biophysica Acta - General Subjects. 2014; 1837(4): 444-450.
- Ricchelli F, Gobbo S, Moreno G, Salet C. Changes of the fluidity of mitochondrial membranes induced by the permeability transition. Biochemistry. 1999 Jul 20;38(29):9295-300. doi: 10.1021/bi9900828. PMID: 10413503.
- Mecocci P, Cherubini A, Beal MF, Cecchetti R, Chionne F, Polidori MC, Romano G, Senin U. Altered mitochondrial membrane fluidity in AD brain. Neurosci Lett. 1996 Mar 29;207(2):129-32. doi: 10.1016/0304-3940(96)12509-x. PMID: 8731438.
- García JJ, Piñol-Ripoll G, Martínez-Ballarín E, Fuentes-Broto L, Miana-Mena FJ, Venegas C, Caballero B, Escames G, Coto-Montes A, Acuña-Castroviejo D. Melatonin reduces membrane rigidity and oxidative damage in the brain of SAMP8 mice. Neurobiol Aging. 2011 Nov;32(11):2045-54. doi: 10.1016/j.neurobiolaging.2009.12.013. Epub 2010 Jan 22. PMID: 20096480.
- Kuter K, Kratochwil M, Berghauzen-Maciejewska K, Głowacka U, Sugawa MD, Ossowska K, Dencher NA. Adaptation within mitochondrial oxidative phosphorylation supercomplexes and membrane viscosity during degeneration of dopaminergic neurons in an animal model of early Parkinson's disease. Biochim Biophys Acta. 2016 Apr;1862(4):741-753. doi: 10.1016/j.bbadis.2016.01.022. Epub 2016 Feb 1. PMID: 26844379.
- Eckmann J, Clemens LE, Eckert SH, Hagl S, Yu-Taeger L, Bordet T, Pruss RM, Muller WE, Leuner K, Nguyen HP, Eckert GP. Mitochondrial membrane fluidity is consistently increased in different models of Huntington disease: restorative effects of olesoxime. Mol Neurobiol. 2014 Aug;50(1):107-18. doi: 10.1007/s12035-014-8663-3. Epub 2014 Mar 18. PMID: 24633813.
- Berg RF, May EF, Moldovera MR. Viscosity ratio measurements with capillary viscometers. Journal of Chemical and Engineering Data. 2014; 59: 116–124.
- Casaretto C, Martínez Sarrasague M, Giuliano S, Rubin de Celis E, Gambarotta M, Carretero I, Miragaya M. Evaluation of Lama glama semen viscosity with a cone-plate rotational viscometer. Andrologia. 2012 May;44 Suppl 1:335-41. doi: 10.1111/j.1439-0272.2011.01186.x. Epub 2011 Jul 6. PMID: 21729143.
- Leclerc GE, Charleux F, Robert L, Ho Ba Tho MC, Rhein C, Latrive JP, Bensamoun SF. Analysis of liver viscosity behavior as a function of multifrequency magnetic resonance elastography (MMRE) postprocessing. J Magn Reson Imaging. 2013 Aug;38(2):422-8. doi: 10.1002/jmri.23986. Epub 2013 Jan 4. PMID: 23293060.
- Sinkus R, Tanter M, Catheline S, Lorenzen J, Kuhl C, Sondermann E, Fink M. Imaging anisotropic and viscous properties of breast tissue by magnetic resonance-elastography. Magn Reson Med. 2005 Feb;53(2):372-87. doi: 10.1002/mrm.20355. PMID: 15678538.
- Fu W, Yan C, Guo Z, Zhang J, Zhang H, Tian H, Zhu WH. Rational Design of Near-Infrared Aggregation-Induced-Emission-Active Probes: In Situ Mapping of Amyloid-β Plaques with Ultrasensitivity and High-Fidelity. J Am Chem Soc. 2019 Feb 20;141(7):3171-3177. doi: 10.1021/jacs.8b12820. Epub 2019 Jan 25. PMID: 30632737.
- Li H, Yao Q, Xu F, Li Y, Kim D, Chung J, Baek G, Wu X, Hillman PF, Lee EY, Ge H, Fan J, Wang J, Nam SJ, Peng X, Yoon J. An Activatable AIEgen Probe for High-Fidelity Monitoring of Overexpressed Tumor Enzyme Activity and Its Application to Surgical Tumor Excision. Angew Chem Int Ed Engl. 2020 Jun 15;59(25):10186-10195. doi: 10.1002/anie.202001675. Epub 2020 Mar 10. PMID: 32155310.
- Zhu X, Wang JX, Niu LY. Aggregation-induced emission materials with narrowed emission band by light-harvesting strategy: fluorescence and chemiluminescence imaging. Chemistry of Materials. 2019; 31 (9): 3573-3581.
- Jiménez-Sánchez A, Lei EK, Kelley SO. A Multifunctional Chemical Probe for the Measurement of Local Micropolarity and Microviscosity in Mitochondria. Angew Chem Int Ed Engl. 2018 Jul 16;57(29):8891-8895. doi: 10.1002/anie.201802796. Epub 2018 Jun 19. PMID: 29808513; PMCID: PMC6338234.
- Park SJ, Shin BK, Lee HW. Asymmetric cyanine as a far-red fluorescence probe for mitochondrial viscosity. Dyes and Pigments. 2020; 174: 108080.
- Sui B, Tang S, Woodward AW. A BODIPY‐based water‐soluble fluorescent probe for mitochondria targeting. European Journal of Organic Chemistry. 2016; 2016 (16): 2851-2857.
- Zhang G, Sun Y, He X, Zhang W, Tian M, Feng R, Zhang R, Li X, Guo L, Yu X, Zhang S. Red-Emitting Mitochondrial Probe with Ultrahigh Signal-to-Noise Ratio Enables High-Fidelity Fluorescent Images in Two-Photon Microscopy. Anal Chem. 2015 Dec 15;87(24):12088-95. doi: 10.1021/acs.analchem.5b02807. Epub 2015 Dec 3. PMID: 26585577.
- Sun W, Shi YD, Ding AX. Imaging viscosity and peroxynitrite by a mitochondria-targeting two-photon ratiometric fluorescent probe. Sensors and Actuators B: Chemical. 2018; 276: 238-246.
- Ren M, Deng B, Zhou K. Single fluorescent probe for dual-imaging viscosity and H2O2 in mitochondria with different fluorescence signals in living cells. Analytical Chemistry. 2017; 89 (1): 552-555.
- Li SJ, Li YF, Liu HW, Zhou DY, Jiang WL, Ou-Yang J, Li CY. A Dual-Response Fluorescent Probe for the Detection of Viscosity and H2S and Its Application in Studying Their Cross-Talk Influence in Mitochondria. Anal Chem. 2018 Aug 7;90(15):9418-9425. doi: 10.1021/acs.analchem.8b02068. Epub 2018 Jul 13. PMID: 29973044.
- Guo L, Zhang R, Sun Y, Tian M, Zhang G, Feng R, Li X, Yu X, He X. Styrylpyridine salts-based red emissive two-photon turn-on probe for imaging the plasma membrane in living cells and tissues. Analyst. 2016 May 23;141(11):3228-32. doi: 10.1039/c6an00147e. PMID: 27160329.
- Guo R , Yin J , Ma Y , Wang Q , Lin W . A novel mitochondria-targeted rhodamine analogue for the detection of viscosity changes in living cells, zebra fish and living mice. J Mater Chem B. 2018 May 14;6(18):2894-2900. doi: 10.1039/c8tb00298c. Epub 2018 Apr 25. PMID: 32254242.
- Chen B, Mao S, Sun Y, Sun L, Ding N, Li C, Zhou J. A mitochondria-targeted near-infrared fluorescent probe for imaging viscosity in living cells and a diabetic mice model. Chem Commun (Camb). 2021 May 4;57(36):4376-4379. doi: 10.1039/d1cc01104a. PMID: 33949482.
- Fang Z, Su Z, Qin W. Two-photon dual-channel fluorogenic probe for in situ imaging the mitochondrial H2S/viscosity in the brain of drosophila Parkinson’s disease model. Chinese Chemical Letters. 2020; 31(11): 2903-2908.
- Dai L, Ren M, Lin W. Development of a novel NIR viscosity fluorescent probe for visualizing the kidneys in diabetic mice. Spectrochim Acta A Mol Biomol Spectrosc. 2021 Jun 5;254:119627. doi: 10.1016/j.saa.2021.119627. Epub 2021 Feb 26. PMID: 33714915.
- Zhang Y, Li Z, Hu W. A mitochondrial-targeting near-infrared fluorescent probe for visualizing and monitoring viscosity in live cells and tissues. Analytical Chemistry. 2019; 91(15): 10302-10309.