More Information
Submitted: 20 December 2019 | Approved: 21 January 2020 | Published: 22 January 2020
How to cite this article: Bassey K, Cosa S. Antiuropathogenic and antioxidant activities of Hypoxis hemerocallidea Lam. extracts and compounds from its taxonomically related species. Arch Pharm Pharma Sci. 2020; 4: 001-009.
DOI: 10.29328/journal.apps.1001020
ORCiD: orcid.org/0000-0001-8178-1221
Copyright License: © 2020 Bassey K, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Antibacterial; Antioxidant; Galpinoside; Hemerocalloside; Uropathogen
Antiuropathogenic and antioxidant activities of Hypoxis hemerocallidea Lam. extracts, and compounds from its taxonomically related species
Kokoette Bassey1* and Sekelwa Cosa2
1Pharmaceutical Sciences Unit, School of Pharmacy, Sefako Makgatho Health Sciences University, Ga-Rankuwa, South Africa
2Division of Microbiology, Department of Biochemistry Genetics and Microbiology, University of Pretoria, Pretoria, South Africa
*Address for Correspondence: Kokoette Bassey, Pharmaceutical Sciences Unit, School of Pharmacy, Sefako Makgatho Health Sciences University. P.O-Box, 60, Medunsa, Ga-Rankuwa, Pretoria, 0204, South Africa, Tel: +27792836751; Email: Edward.bassey@smu.ac.za
Hypoxis hemerocallidea Lam is one of the 43 Hypoxis species in South Africa, marketed extensively as over the counter herbal product for the management of several diseases. The plant commercial products link efficacy to hypoxoside 4, its aglycone (rooperol), or β-sitosterol. This study investigated antibacterial and anti-oxidant activities of four other molecules and two extracts from the Hypoxis plant. Visual antioxidant limit of detection and free radical scavenging activities of test samples were determined using 20 mM hydrogen peroxide, 0.4 mM DPPH or ferric reducing antioxidant power assay. Quantitative free radical was determine by spectrophotometric method while antibacterial activity was evaluated using MICs against uropathogens: S. aureus (ATCC25923), S. marcescens (ATCC 14041), P. aeruginosa (ATCC 9721), P. mirabilis (ATCC 33583) and E. coli (ATCC 10536).
Anti-oxidant activities visual limit of detection of 0.06 mg/mL and percentage free radical scavenging activity (IC50 = 0.048 - 0.032 mg/ml) for pure compounds 1 - 4 and (IC50 = 0.037 - 0.039 mg/ml) for extracts were obtained. The reducing power IC50 ranged between 0.15 - 0.23 mg/ml for extracts and 0.11-0.35 mg/ml for standards. Antibacterial potentials show a noteworthy to moderate MIC values of 0.20 - 1.56 mg/ml against S. aureus (ATCC 25923), S. marcescens (ATCC 14041), P. aeruginosa (ATCC 9721), P. mirabilis (ATCC 33583) and E. coli (ATCC 10536). Galpinoside 5, hemerocalloside 3 and curculigoside C 2 are the other active compounds in Hypoxis plant.
Hypoxis hemerocallidea Lam. (H. hemerocallidea) of the Hypoxidacea genus is a perennial herbal and medicinal plant that is widely distributed in sub-Saharan Africa, in particular the Southern part of Africa. Anecdotal evidence accentuates the infusions and decoctions of the plant in South African traditional health care system. Worthy of note is the use of the plant corm polar extracts in the management of disease such as urinary tract infections, common cold, flu, nausea [1], prostate hypertrophy [2]. In addition to its traditional uses, in vitro and in vivo studies have indicated the plant extracts to possess anti-oxidant and antidiabetic [3], anticonvulsant, antibacterial, antidiarrhoeal, immunomodulatory, uterolytic, bronchorelaxant, CD4 count reduction pharmacological properties [4]. Several phytochemicals including but not limited to hypoxoside 4, dehydroxy hypoxoside, bis-dehydroxy hypoxoside [5], nyasoside, nyasicoside, mononyasine A, mononyasine B and nyaside [6], interjectin, geraniol 1, obtuside A, obtuside B [7], zeatin and its glycoside [8], have been isolated from the plant. Bassey and co-workers [9] reported seven other compounds including galpinoside 5, orcinal glycoside, geraniol glycoside 1 and curculigoside C 2 from taxonomically related H. colchicifolia and H. galpinii. However, the acclaimed medicinal properties of the polar extracts of the plant are link to the prodrug hypoxoside 4, its aglycone-rooperol or β-sitosterol only. Hence, the present study investigated the antioxidant activities of galpinoside 5, hemerocalloside 3, curculigoside C 2, and geraniol glycoside 1, the other molecules present in H. hemerocallidea or related species. The antibacterial activities of these molecules against selected uropathogens were also investigated. A number of bacterial pathogens associated with UTIs include Staphylococcus aureus, Proteus mirabilis, Pseudomonas aeruginosa, Klebsiella pneumonia, Klebsiella oxytoca, Citrobacter freundii, Enterobacter cloacae, and Serratia marcescens among many others. E. coli is the most common etiological agent of urinary tract infections (UTIs) in both hospital and community acquired infections. According to Bitew, et al. [10], uropathogens associated infections are implicated to greater than or equal to 6 million outpatient visits and 479,000 hospitalizations annually in the United States alone. Whereas the resultant treatment costs are estimated to be greater than 2.47 billion USD. Consequently, the UTIs are associated with the rise in antibiotic resistance in the hospital setting [11]. The profile of bacterial uropathogens have changed drastically over the years due to their evolving adaptive strategies contributing to antibiotic resistance [10,12,13]. For this reason, it becomes imperative to search and screen for more antimicrobial potentials against resistant pathogens. The present study thus explored the potential antibacterial activities of the H. hemerocallidea extracts and compounds 1 - 5 isolated from it or taxonomically related H. colchicifolia and H. galpinii against the mentioned bacterial uropathogens.
Reagents and solvents
All solvents were of analytical (AR) grade. Methanol (MeOH), ethanol (EtOH), acetone, chloroform (CHCl3), sulphuric acid, ascorbic acid, 2,2-dipicryl-diphenyl hydrazyl (DPPH), Amoxicillin, ciprofloxin, Luria Bertani (LB) soft agar or Mueller Hinton (MH) agar were purchased from either Rochelle chemicals and Lab equipment CC, South Hills or Sigma-Aldrich, Johannesburg South Africa.
Plant materials and standard compounds
Crude water and methanol extracts of H. hemerocallidea as well as five pure compounds hypoxoside 4, galpinoside 5, hemerocalloside 3, curculigoside C 2 and geraniol glycoside 1 with UPLC-MS purity of 95% were obtain from a previous study. Possible degradation of each standard compound was confirmed using TLC analysis of each compound developed in chloroform: methanol: water (CHCl3: MeOH: H2O 70:30:2 v/v/v). The retardation factor (Rf) value for each compound was compared with literature for the same compounds analyzed using the same mobile phase [9].
Qualitative free radical scavenging (Antioxidant) DPPH Dot blot staining assay
Visual limit of detection determination: The qualitative free radical scavenging activity of the test samples and controls were investigated using the method described by Mon and co-workers [14] with minor modifications. Using a glass Pasture pipette, 5 drops of the water and methanol extracts of H. hemerocallidea as well as standard compounds (hypoxoside 4, galpinoside 5, hemerocalloside 3, curculigoside C 2 and geraniol glycoside 1) were separately spotted on a 5 x 5 cm silica gel pre-coated glass plates (Silica gel 60 F254, Merck, Germany) and dried using a creek of Table fan air. The modifications included the sample concentrations spotted as follows: 1.0, 0.5, 0.1, 0.09, 0.08, 0.07, 0.06, 0.05 and 0.01 mg/mL of extracts, standards and vitamin C (positive control). A 0.4 mM (1.57 g DPPH/10 mL MeOH) solution was prepared immediately and smeared on the Dot blot TLC plate using a cotton wool.
Quantitative determination of antioxidant activity (in vitro DPPH, Hydrogen peroxide free radical scavenging assay and Ferric reducing antioxidant power): The quantitative anti-oxidant activity (AOXA or %DPPH) were examine using spectrophotometry measurement of 1,1-diphenyl-dipicryl hydrazyl (DPPH) free radical scavenging assay. 1.0 mL of 1.0, 0.5, 0.1, 0.08, and 0.05 mg/mL of water, methanol extracts of H. hemerocallidea, and the 5 standards (1-5) were mix with 1 mL of 0.4 mM DPPH solution in methanol. The mixtures were allow to react at room temperature in the dark for 30 minutes. Blank solutions of 1.0 mL DPPH + 1.0 mL methanol were use as the negative control while L-ascorbic acid (Vitamin C) and 0.4 Mm of the DPPH was the positive control. The tests were carried in triplicate and mean values were recorded. Hydrogen peroxide free radical scavenging assay was done with the same protocol using 20 mM of 30% w/v H2O2 mixed with PBS (pH = 7.67). Two milliliters (2.0 ml) of the hydrogen peroxide mixed with 1.0 ml each of the serially diluted (1.0, 0.5, 0.1, 0.08, and 0.05 mg/mL) test samples solution were separately allowed to react in the dark before reading the absorbance. The negative control was H2O2-PBS solution with no test samples while vitamin C was the positive control. The decrease in absorbance was measured at 518 nm or 230 nm for DPPH and the H2O2 assays respectively using spectrophotometer (spectrophotometers Nanocolor® uv/vis, Macherey-Nagel GmbH & Co. KG, Germany). The ferric reducing power antioxidant (FRAP) assay was conducted using test extracts, standards and control. The working plant extracts, standards and control solutions were prepared by diluting the 1 mg/ml stock solution to 1.0, 0.5, 0.1, 0.08, and 0.05 mg/mL. A milliliter (1.0 ml) of the working solution of the plant extracts, standards and control was mixed with 2.5 ml of 0.2 M phosphate buffer (pH 6.60) and 2.5 ml of a 1% (w/v) solution of potassium ferricyanide (K3[Fe(CN)6]). The mixture was vortexed, and then incubated in water bath at 50 °C for 20 min. Thereafter, 2.5 ml of a 10% (w/v) trichloroacetic acid was added, and then centrifuged at 3000 rpm for 10 min. A volume of 2.5 mL of the supernatant was transferred into a test tube and mixed with and 0.5 mL of a 0.1% (w/v) solution of ferric chloride solution. The mixture was well vortexed and the increasing absorbance measured at 700 nm using the afore mentioned spectrophotometer. Values obtained were converted to percentage antioxidant activity (%DPPH) using the formula:
% DPPH scavenging capacity = (A0 – As / A0) x 100 (1)
Where A0 indicates absorbance of the negative control 1.0 mL of DPPH solution + 1.0 ml of methanol, 2.0 ml of H2O2 PBS or 2.5 mL of the supernatant + 0.5 mL ferric chloride solutions and As represents the absorbance of the positive control - 1.0 ml of DPPH solution + 1.0 ml, 2.0 ml H2O2-PBS solution or 2.5 mL supernatant + 0.5 mL of ferric chloride mixed with 1.0 ml of extracts, standards or vitamin C solution.
Dot Blot stain antimicrobial bio-autography: The Dot Blot stain for antimicrobial bio-autography was conducted as described by Mon and co-workers [14] with modifications. The 5 x 5 cm silica gel pre-coated glass plates (Silica gel 60 F254, Merck, Germany) was diagonally divided into two equal halves. Five glass Pasture pipette drops of 1.0 mg/mL of the water, methanol extracts or the standards compounds (hypoxoside 4, galpinoside 5, hemerocalloside 3, curculigoside C 2 and geraniol glycoside 1) were spotted at the bottom corner of the plate. Ciprofloxacin and amoxicillin (0.1 mg/mL) positive control were spotted at the top corner of the TLC plate. The plates were dried using a creek of Table fan air. About 5 mL of the Luria Bertani (LB) soft agar or Mueller Hinton (MH) agar solution seeded with 0.5 mL of S. aureus (ATCC 25923), S. marcescens (ATCC 14041), P. aeruginosa (ATCC 9721), P. mirabillis (ATCC 33583) and E. coli (ATCC 10536) prepared overnight and standardized at 0.05 McFarland standard, poured on the prepared Dot blot TLC plate. The plates were incubated at 37 ⁰C for 24 hours, thereafter zones of inhibition appearing as creamy white blots or clear on a red (S. marcescens) or creamy white (S. aureus, P. mirabillis and E. coli) or green-yellowish (P. aeruginosa) pigment backgrounds were observed .
Antibacterial minimum inhibition (MIC) by 96-well assay: The bacterial cultures of S. aureus (ATCC 25923), S. marcescens (ATCC 14041), P. aeruginosa (ATCC 9721), P. mirabillis (ATCC 33583) and E. coli (ATCC 10536) were grown for 18-24 hours at 37°C and thereafter adjusted to OD600 nm 0.08-0.1. The 96-well polystyrene microtiter plate assay was used to determine the lowest concentration of extracts that inhibits bacterial growth [15]. The two-fold serial dilution microplate method was used to determine the MIC values. Briefly, aliquots (100 μl) of 25 mg/mL crude extracts and 1 mg/mL isolated pure standard compounds were serially diluted with Mueller Hinton (MH) broth in each well. A 100-μl aliquot of standardized bacterial suspension were added to each well. Mueller Hinton broth and 1% DMSO were use as negative and solvent control, respectively. Ciprofloxacin (0.01 mg/mL) and amoxicillin (0.01 mg/mL) were use as positive controls. Para-iodonitrotetrazolium (INT, 0.2 mg/ml) 40 μL was added after 24 hours of incubation, thereafter incubated for further 30 min to 1 hour until optimal colour development was observed. Tests were carried out in triplicate. The MIC was recorded as the lowest concentration of the extract/isolated standard compound that inhibited bacterial growth as described by Pauw and Eloff [15].
Standard compounds purification and identification
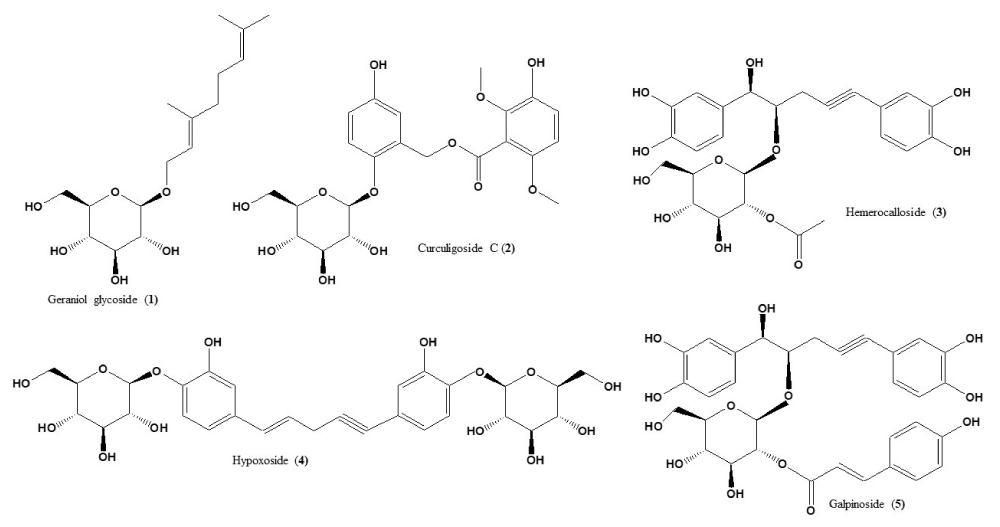
The stability of each standard compound used in this study was confirmed using simple TLC developed in CHCl3:MeOH:H2O (70:30 2 v/v/v). Single compact spot for each compound was obtained with Rf (0.76, 0.50, 0.47, 0.45, and 0.43 for geraniol glycoside 1, curculigoside C 2, hemerocalloside 3, hypoxoside 4 and galpinoside 5, - Figure 1. The Rf values matched those previously reported using the same mobile phase [9].
Figure 1: Structures of geraniol glycoside 1, curculigoside C 2, hemerocalloside 3, hypoxoside 4 and galpinoside 5, standard compounds used in this study.
Visual limit of detection by qualitative free radical scavenging (Antioxidant) DPPH
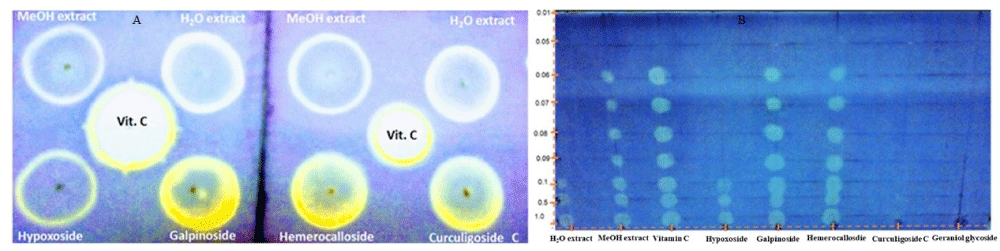
Dot Blot staining assay: A dot-blot staining test was preferred over the classical TLC bio-autography to evaluate the qualitative anti-oxidant activity of the Hypoxis extracts, using standards (hypoxoside 4, galpinoside 5, hemerocalloside 3, curculigoside C 2, geraniol glycoside 1) and L-ascorbic acid (vitamin C) as a positive control. Result obtained (Figure 2 top) revealed that galpinoside 5 and hemerocalloside 3 exhibit free radical scavenging properties that is comparable to that of ascorbic acid judging by the white-yellow bleaching of the purple DPPH background.
Established by the thickness of the white-yellow coloration, the Hypoxis hemerocallidea methanol and water extracts also indicated relatively moderate anti-oxidant activity than hypoxoside 4, the commercial molecules from most Hypoxis species (Figure 2 top). The limit of detection, LOD of any analytical instrument or method is often determine by injecting very low concentration, (usually ≤ 1 mg/mL) of an anlyte into the instrument and using area under the curve (AUC) to determine the lowest concentration that can be detected. This study, implemented this idea by spotting 1.0 - 0.001 mg/mL of Hypoxis hemerocallidea water and methanol extracts, standard galpinoside 5, hemerocalloside 3, curculigoside C 2 and geraniol glycoside 1 (Figure 2 bottom) on a TLC glass plate to visualize their anti-oxidant activities limit of detection. Hypoxoside 4, indicated moderate radical scavenging activity with a visual limit of detection value of 0.1 mg/mL, a value equal to that of the Hypoxis hemerocallidea water extract (Figure 2 bottom). The anti-oxidant activity of galpinoside 5, hemerocalloside 3 standards and methanol extract has never been reported. Our findings revealed DPPH free radical scavenging visual LOD values of 0.06 mg/mL comparable to the positive control vitamin C. Whereas traces of curculigoside C 2 were observed with visual LOD at 1 mg/mL with the cream discoloration of the purple DPPH fading off in a matter of seconds, geraniol glycoside 1 was not visually detected in the concentration range studied.
Figure 2: Dot Blot stains indicating anti-oxidant activities of Hypoxis hemerocallidea methanol and water extracts, standards and vitamin C against DPPH (Top) and visual anti-oxidant LOD determination using the Dot blot staining test of Hypoxis hemerocalloside extracts, standards and positive control at concentration 1.0-0.001 mg/mL (bottom).
Quantitative determination of antioxidant activity (in vitro DPPH and H2O2 free radical scavenging assay)
The in vitro quantitative anti-oxidant activity was determine by analyzing the same extracts, standards and positive control used for the qualitative DPPH visual LOD assay. This was important to further confirm the exact antioxidant activity of H. hemerocallidea methanol extract, galpinoside 5, hemerocalloside 3 and vitamin C with the same visual anti-oxidant LOD of 0.06 mg/ml.
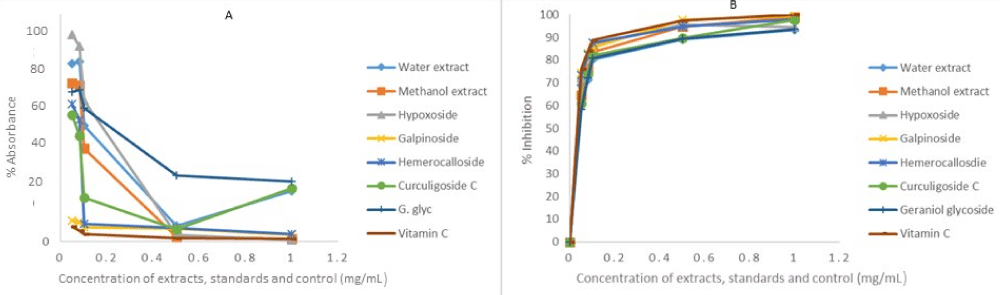
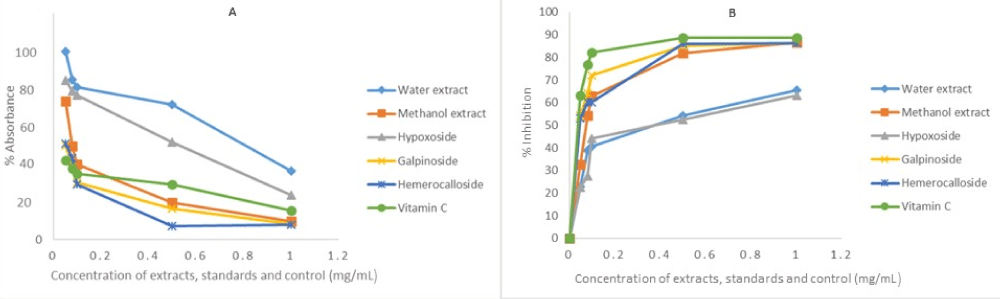
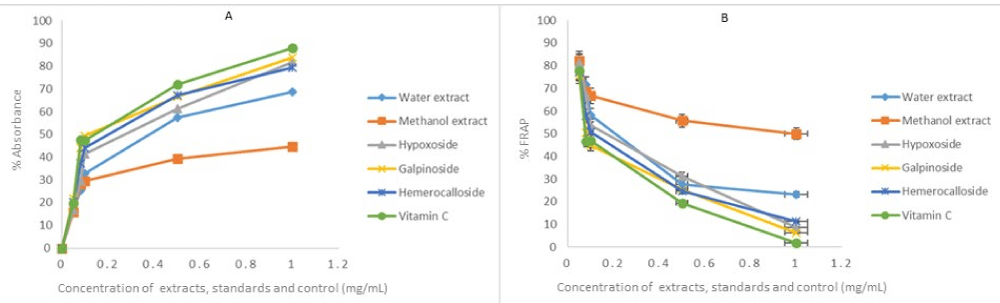
The same reason also necessitated the in vitro anti-oxidant activity evaluation of H. hemerocallidea water extract and hypoxoside 4 that exhibited identical visual anti-oxidant LOD of 0.1 mg/mL. Figure 3 depicts the absorbance versus concentration function for the Hypoxis extracts, standards and vitamin C. The reduction in the absorbance of DPPH (Figure 3A) and the H2O2 (Figure 4A) is an indication of the degree to which the extract and standard compounds can inhibit the DPPH and H2O2 radical generation activity [14]. This is associated with the free radical scavenging activities of tested samples. Conversely, the increasing absorbance for the FRAP assay (Figure 5A) further validated the antioxidant potentials of test samples. The results from the three antioxidant assays concurs with results obtained for the visual LOD of the qualitative anti-oxidant activity obtained using the Dot Blot assay as galpinoside 5, displays a free radical scavenging power that is comparable with ascorbic acid.
Figure 3: DPPH absorbance-concentration function (A) percentage inhibition of DPPH at 518 nm of H. hemerocallidea extracts (water, methanol), standards and vitamin C. Each value is expressed as Mean ± standard deviation (n = 3)
Figure 4: H2O2 absorbance-concentration function (A) percentage inhibition of H2O2 at 230 nm of H. hemerocallidea extracts (water, methanol), standards and vitamin C. Each value is expressed as Mean ± standard deviation (n = 3).
Figure 5: Fe3+ to Fe2+ reducing power of test H. hemerocallidea extracts, standards and vitamin C. Each value is expressed as Mean ± standard deviation (n = 3).
The percentage free radical scavenging activity of the test H. hemerocallidea extracts, standards and vitamin C were evaluated to further validate the degree to which the extract and standard compounds can inhibit the DPPH and H2O2 radical generation activity as well as reduce Fe3+ to Fe2+. As indicated (Figure 3B-5B and Table 1), galpinoside 5, exhibited potent anti-oxidant activity with IC50 = 0.030 (DPPH), 0.04 (H2O2) mg/mL) with a reducing power (IC50 = 0.17) comparable to that of vitamin C, with IC50 = 0.028 (DPPH), 0.038 (H2O2) (0.11) mg/mL respectively. This was followed by hemerocalloside 3, and methanol extracts with average IC50 = 0.041 mg/mL) and IC50 = 0.056 mg/mL respectively for both free radical test methods. Whereas curculigoside C 2 has DPPH IC50 = 0.045 mg/mL and hypoxoside 4 (IC50 = 0.037) one-to-one, geraniol glycoside 1, exhibited the lowest percentage antioxidant activity with (IC50 = 0.048 mg/mL).
| Table 1: Calculated IC50 indicating the percentage free radical activity of Hypoxis water, Methanol extracts, standards galpinoside 5, hemerocalloside 3, curculigoside C 2, geraniol glycoside 1 and vitamin C positive control. | |||
| Anlyte | Fee radical scavenging Assay | ||
| DPPH IC50 (mg/mL) | H2O2 IC50 (mg/mL) | FRAP IC50 (mg/mL) | |
| H. hemerocallidea water extract | 0.037 ± 0.10 | 0.08 ± 1.29 | 0.23 ± 2.58 |
| H. hemerocallidea methanol extract | 0.039 ± 1.40 | 0.073 ± 2.04 | 0.15 ± 1.25 |
| Hypoxoside (4) | 0.037 ± 0.97 | 0.09 ± 1.40 | 0.35 ± 2.87 |
| Galpinoside (5) | 0.030 ± 1.40 | 0.04 ± 1.30 | 0.17± 2.62 |
| Hemerocalloside (3) | 0.032 ± 0.98 | 0.05 ±1.44 | 0.20 ± 2.64 |
| Curculigoside C (2) | 0.045 ± 1.42 | Nd | Nd |
| Geraniol glycoside (1) | 0.48 ± 1.41 | Nd | Nd |
| Vitamin C | 0.028 ± 0.97 | 0.038 ± 1.08 | 0.11 ± 2.91 |
| Nd: Not determined due to limited test standards. | |||
Dot blot stain antimicrobial bio-autography
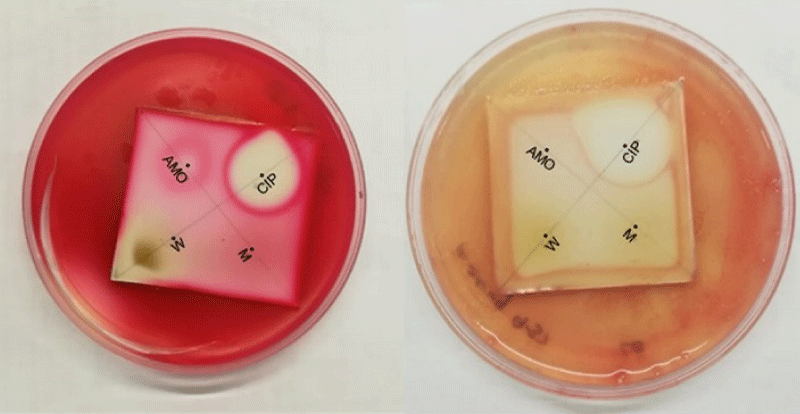
The Dot Blot staining assay (Figure 6) revealed that only the water extract indicated positive change for inhibition of S. marcescens as represented by the zone of inhibition in the area of water extract. However, both methanol and water extracts of H. hemerocallidea showed inhibitory activity against S. aureus, S. marcescens and P. aeruginosa. From the standards compounds, only curculigoside C 2, inhibited E. coli with an MIC of 3.13 mg/mL. Hypoxoside 4, galpinoside 5, hemerocalloside 3 and geraniol glycoside 1, exhibited no antimicrobial activities.
Figure 6: Selected anti-microbial Dot Blot staining bioautography. Left: S. marcescens and right: P aeruginosa. CIP (ciprofloxacin 0.01 mg/ml), AMO (amoxicillin 0.01 mg/ml), W (water extract of H. hemerocallidea) and M (methanol extract of H. hemerocallidea).
Minimum Inhibitory Concentrations (MICs)
The MIC values for test samples (Hypoxis water, methanol extracts, standards galpinoside 5, hemerocalloside 3, curculigoside C 2, geraniol glycoside 1) that exhibited activities against the test pathogens (P. aeruginosa, S. aureus, S. marcescens, P. mirabilis and E. coli) are presented in table 2. From these findings, the H. hemerocallidea water extract showed potent and noteworthy activities of ≤ 1 mg/mL [17] against all test pathogens except for P. aeruginosa. H. hemerocallidea water extract (MIC value of 0.78 mg/mL) inhibited S. aureus and S. marcescens, while 0.39 mg/mL inhibited P. mirabilis and the best MIC of 0.20 mg/mL against E. coli was also observed. The MIC value of 1.56 mg/mL of the same extract at least inhibited P. aeruginosa. In contrast, the MIC values for methanol extract ranged between 1.56 and 3.13 mg/mL were not as “noteworthy” because the extracts exhibit MIC > 1 mg/mL [17]. The methanol extract (MIC = 1.56 mg/mL) was noted against S. aureus, while S. marcescens, P. aeruginosa, P. mirabilis and E. coli were inhibited at MIC = 3.13 mg/mL. All the isolated test pure compounds inhibited the test pathogens by a concentration of 0.25 mg/mL except for curculigoside C 2, which exhibited MIC = 0.13 mg/mL against E. coli. This observation further confirms that E. coli was better susceptible to extracts and compounds from H. hemerocallidea. When compared to the ciprofloxacin and amoxicillin (MIC range of 0.003 - 0.25 mg/mL and 0.01 - 0.25 mg/mL respectively); H. hemerocallidea water extract (MIC 0.20 mg/mL against E. coli), curculigoside C 2, (MIC range of 0.13 - 0.25 mg/mL) were recorded. Similar results were observed for galpinoside 5, hemerocalloside 3, and hypoxoside 4 (all three with MIC of 0.25 mg/mL). These results suggest ≤ 0.25 mg/mL as the threshold MIC value for the Hypoxis plant polar extracts, isolated standards vis-a-vis the commercial antibiotics like ciprofloxacin and amoxicillin.
| Table 2: Minimum inhibitory concentrations (mg/mL) for Hypoxis hemerocallidea water, methanol extracts pure isolated compounds and positive control against selected uropathogens. | |||||
| Test samples | MIC (mg/mL) | ||||
| Plant extracts | S. aureus | S. marcescens | P. aeruginosa | P. mirabilis | E. coli |
| Water | 0.78 | 0.78 | 1.56 | 0.39 | 0.20 |
| Methanol | 1.56 | 3.13 | 3.13 | 3.13 | 3.13 |
| Compounds | |||||
| Galpinoside | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Hemerocalloside | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Hypoxoside | 0.25 | 0.25 | 0.25 | 0.25 | |
| Curculigoside C | 0.25 | 0.25 | 0.25 | 0.25 | 0.13 |
| Controls | |||||
| Ciprofloxacin | 0.003 | 0.003 | 0.003 | 0.003 | 0.25 |
| Amoxicillin | 0.01 | 0.25 | 0.25 | 0.25 | 0.25 |
| 1% DMSO | 6.25 | 6.25 | 6.25 | 6.25 | 6.25 |
The isolated standards compounds were identified using literature references as hypoxoside 4, galpinoside 5, hemerocalloside 3 [9], curculigoside C 2 and geraniol glycoside 1 [16]. Hypoxoside 4 is a pentyne derived natural pro-drug present in every Hypoxis species. However, hypoxoside 4, could be hydrolyz to rooperol by the action of glucosidase enzyme. Although the compound hypoxoside 4 is now commonly isolated in Hypoxis hemerocallidea as its major compound, it was initially isolated from other Hypoxis species including H. obtusa. Hypoxoside 5 is available on the market as vital medicinal compound [18]. Galpinoside 5, and others were previously isolated from South African Hypoxis species, adding to the list of secondary metabolites from the genus. Although these compounds were identified, no evaluation of their antibacterial and antioxidant activities were conducted.
This study hereby identifies galpinoside 5, hemerocalloside 3 and curculigoside C 2 as polar phenolic compounds isolated from polar extracts of Hypoxis plant as the compounds that possess the anti-oxidant activity. The good antioxidant activity (LOD of 0.01 mg/mL) of galpinoside 5, and hemerocalloside 3, standard compounds whose anti-oxidant activity has never been reported alongside the methanol extract of the plant, is comparable to the positive control vitamin C. Whereas curculigoside C 2, has a moderate visual antioxidant activity at a concentration of 1 mg/mL and geraniol glycoside 1, had none. This results mirrors that reported by Mannathoko and co-workers [19] for similar antioxidant activity of 0.05 mg/mL for methanol extract of H. hemerocallidea corm and vitamin C (ascorbic acid). The mechanism of the anti-oxidant activity of galpinoside 5, and hemerocalloside 3, could be via the diacatechol-type (rooperol or nordihydroguaiaretic acid) pathway previously reported by other groups [20 - 21]. Most compounds with good antioxidant activities usually have 3,4-dihydroxy groups that is responsible for the reduction of the free radicals from the DPPH or H2O2 solution. The same reasons is also responsible for the relatively poor antioxidant activity of curculigoside C 2, observed at 1 mg/mL with a 3-hydroxy group in its structure and the zero activity of geraniol glycoside 1, being a consequence of the lack of phenyl and hydroxyl group in its structure. The synergistic presence of galpinoside 5, hemerocalloside 3 and curculigoside C 2, with catechol-type or rooperol-type structure in the methanolic extract of H. hemerocallidea should be responsible for its relatively better anti-oxidant activity over the water extract and hypoxoside 4. The two sugar units in hypoxoside 4, would reduce the anti-oxidant activity. Work is currently underway in our laboratory to compare the activity of the aglycones of the active antioxidant compounds to that of rooperol, an aglycone with better antioxidant than the parent hypoxoside 4, prodrug.
When quantitative determination of antioxidant activity (in vitro DPPH, H2O2 free radical scavenging assay and ferric reducing power) and percentage antioxidant activities were performed, results (Figure 3B - 5B) underscores 0.5 mg/mL as the optimal concentration for best free radical scavenging properties for the methanol extract, galpinoside 5, hemerocalloside 3 and vitamin C on the one hand and water extract and hypoxoside 4 on the other. This is due to the absorbance of the DPPH, H2O2, and ferric chloride reducing power at this concentration is lowest. This would imply that commercial products and muti that contain Hypoxis extracts and pure antioxidant compounds like galpinoside 5 and hemerocalloside 3 must maintain this optimal concentration for optimal anti-oxidant activity ceteris Paribus. The methanol extract, galpinoside 5 and hemerocalloside 3 has a quantitative free radical scavenging activity that is comparable to that of the positive control vitamin C. However, galpinoside 5 would be the compound with the best comparable anti-oxidant activity to vitamin C. The other analytes and vitamin C has linear absorbance after this concentration to indicate a possible similarity in their free radical scavenging potential compared to vitamin C to agree with results previously published [19-20]. The results obtained for the percentage free radical scavenging properties were 87.98% (IC50 = 0.030 mg/mL) for galpinoside 5, compared with 89.93% (IC50 = 0.028 mg/mL) of vitamin C and 87.20% (IC50 = 0.037 mg/mL) for hemerocalloside 3, supports those previously reported [19, 22] which highlighted that polar extracts of the fresh leaf and corms has high anti-oxidant activity associated with highly polar constituents. This inference is justified from the perspective of galpinoside 5 and hemerocalloside 3 being highly polar compounds isolated from Hypoxis plant with solubility in methanol and water. As mentioned by Katerere and Ellof [22], polar phenolic compounds are responsible for the anti-oxidant activity of Hypoxis hemerocallidea extract. From Figure 2 (bottom), the moderate antioxidant activity of hypoxoside 4 and the H. hemerocallidea water extract with a visual limit of detection value of 0.1 mg/mL is due to compounds lacking dicatechol-type structure.
The poor to moderate antioxidant activity of the water extract and hypoxoside 4, were complimented by the noteworthy MIC of 0.39 and 0.20 mg/mL against P. mirabilis and E. coli respectively, and ≤ 0.25 mg/mL for hypoxoside 4 and water extract. Bacterial growth inhibition of E. coli by H. hemerocallidea water extract was marginally greater than the activity of the commercial ciprofloxacin with an MIC value of 0.25 mg/mL. This could be a function of improved synergy between the compounds in the water extract as compared to the methanol extract. Although the extraction solvent polarity decreases, the inhibition of the organisms by the polar extract of South African H. hemerocallidea increases. As example, the acetone < ethanol demonstrate MIC 2.5 mg/mL > 0.31 mg/mL [23] and MeOH < H2O with 3.13 mg/mL > 0.20 mg/mL (present study). Matotoka and Masoko [23] also documented H. hemerocallidea acetone extract with 2.5 mg/mL, 0.63 mg/mL, and 0.03 mg/mL MIC values against P. aeruginosa, S. aureus and E. faecalis, respectively.
Galpinoside 5, hemerocalloside 3 and hypoxoside 4 with MIC value of 0.25 mg/mL for all the test pathogens performed equally as ciprofloxacin against E. coli and amoxicillin against S. marcescens, P. aeruginosa, P. mirabilis and E. coli (MIC = 0.25 mg/mL). Overall, curculigoside C 2, exhibited the prominent noteworthy antibacterial property against E. coli (MIC = 0.13 mg/mL). This activity was significantly improved than ciprofloxacin and amoxicillin (MIC = 0.25 mg/mL). The rationalization for potent results obtained with curculigoside C against all the test pathogens and the possible mechanism of action includes the structure of curculigoside C; where the presence of hydroxyl groups OHs at C-3, C-3’ positions and the two vicinal oxygen-bearing groups at the benzene ring [24] when compared to ciprofloxacin and amoxicillin would attest to this. This concentration (0.25 mg/mL) is clinically important for manufacturers and users of herbal medicines formulated from H. hemerocallidea polar extract and standards compounds isolated from the plant extracts. The superfluity of health benefits available for consumers of H. hemerocallidea products in particular and other Hypoxis species in general should not be merely associated to hypoxoside 4, (or rooperol) and β-sitosterol. Justifiably, additional compounds like galpinoside 5, hemerocalloside 3 and curculigoside C 2 present in the polar extract particularly possess the biological activities for which Hypoxis plant is indicated for in the present study.
The authors thank the Research and Development Grant (D113) of the Sefako Makgatho Health Sciences University for funding.
- Mills E, Cooper C, Seely D, Kanfer I. African herbal medicines in the treatment of HIV: Hypoxis and Sutherlandia. An overview of evidence and pharmacology. Nutr J. 2005; 4: 1-6. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15927053.
- Drewes SE, Hall HJ, Learmonth RA, Upfold UJ. Isolation of hypoxoside From Hypoxisrooperi and synthesis of [E]-1,5-bis[3,4-dimethoxyphenyl] pent-4-en-1-yne. Phytochem. 1984; 23: 1313-1316. https://www.sciencedirect.com/science/article/abs/pii/S0031942200804495
- Oguntibeju OO, Meyer S, Aboua YG, Goboza M. Hypoxis hemerocallidea Significantly Reduce hyperglycaemia and hyperglycaemic-induced oxidative stress in the liver and kidney tissues Of streptozotocin-induced diabetic male Wistar rats. Evid.-Based Complementary Altern Med. 2016; 2016: 1-10.
- Ojewole JAO. Antinociceptive, anti-inflammatory and anti-diabetic properties Of Hypoxis hemerocallidea Fisch., C.A. Mey. (Hypoxidaceae) corm [African potato] Aqueous extract in mice and rats. J Ethnopharmacol. 2006; 103: 126-134. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16191469
- Laporta O, Perez-Fons L, Mallavia R, Caturla N. Isolation, characterization and antioxidant capacity assessment of the bioactive compounds derived from Hypoxis rooperi corm extract (African potato). Food Chem. 2007; 101: 1425-1437. https://www.sciencedirect.com/science/article/pii/S0308814606002925
- Marini-Bettolo GB, Nicoletti PM, Galeffi C. Research on African medicinal plants-II: Hypoxoside a new glycoside with uncommon structure from Hypoxis obtusa busch. Tetrahedron. 1982; 38: 1683-1687. https://www.sciencedirect.com/science/article/abs/pii/0040402082801476
- Galeffi C, Multari G, De Vicente Y, Messan I, Nicolleti M, Marini-Bettolo GM. Two New Glucosides from Hypoxis obtusa: Obtuside A and Obtuside B. Planta medica. 1989; 55: 318-320. https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-2006-962019?device=mobile
- Van Staden J. Constituents of Hypoxis rooperi, a valuable medicinal plant in South Africa. Deutsche Apotheke Zeitung. 1989; 33: 460-464.
- Bassey K, Viljoen A, Combrinck S, Choi YH. New phytochemicals From the corms of medicinally important South African Hypoxis species. Phytochem Lett. 2014; 10: lxix–lxxv. https://www.sciencedirect.com/science/article/abs/pii/S1874390014001748
- Bitew A, Molalign T, Chanie M. Species distribution and antibiotic susceptibility profile of bacterial uropathogens among patients complaining urinary tract infections. BMC InfectDis. 2017; 17: 1-8. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28962545
- Tabibian JH1, Gornbein J, Heidari A, Dien SL, Lau VH. et al. Uropathogens and host characteristics. J Clin Microbiol. 2008; 46: 3980-3986. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18842936
- Foxman B, Barlow R, D'Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000; 10: 509-15. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11118930
- Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and Economic costs. Am J Med. 2002; 113: 5-13. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12113866
- Mon MM, Maw SS, Oo ZK. Quantitative determination of free radical scavenging activity and antitumor activity of some Myanmar herbal plants. Int Sch Sci Res Inn. 2011; 5: 92-98. http://www.ipcbee.com/vol11/22-T046.pdf
- Pauw E, Eloff JN. Which tree orders in southern Africa have the highest antimicrobial activity and selectivity against bacterial and fungal pathogens of animals? BMC Complement Altern Med. 2014;14: 1-12. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25164197
- Bredenkamp MW, Drewes SE, Wentler GL. A geraniol glycoside from Hypoxisacuminata. Phytochem. 1989; 28: 263-265. https://www.sciencedirect.com/science/article/abs/pii/0031942289850538
- Van Vuuren S. Antimicrobial activity of South African medicinal plants. J Ethnopharmacol. 2008; 119: 462-472. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18582553
- Nsibande BE, Gustavsson K, Zhu L. Analysis of health-associated Phytochemical compounds in seven Hypoxis species. AJPS. 2018; 9: 571-583. https://pdfs.semanticscholar.org/949d/3e30c54269cc6579ae3cfcb7e8c271b9ddef.pdf
- Mannathoko N, George S, Souda S, Chabaesele K, Goercke I. An in vitro Analysis of the antioxidant and antimicrobial properties of the methanol extract of Hypoxis hemerocallideacorm (MEHHC) from Botswana. MRJI. 2017; 19: 1-12. https://scite.ai/reports/an-in-vitro-analysis-of-the-N593dw
- Lundberg WO, Halvorson HO, Burr GO. The antioxidant properties of nordihydroguaiaretic acid. Oil & Soap. 1994; 21: 33-35. https://link.springer.com/article/10.1007/BF02593156
- Nair VDP, Dairam A, Agbonon JT, Arnason BCF, Kanfer I. Investigation of the antioxidant activity of African potato (Hypoxis hemerocallidea). J Agric Food Chem. 2007; 55: 1707-1711. https://pubs.acs.org/doi/abs/10.1021/jf0619838
- Katerere DR, Eloff JN. Anti-bacterial and anti-oxidant activity of Hypoxis hemerocallidea (Hypoxidaceae): Can leaves be substituted for corms as a Conservation strategy? SAJB. 2008; 74: 613-616. https://www.sciencedirect.com/science/article/pii/S025462990800197X
- Matotoka MM, Masoko P. Evaluation of herbal concoctions sold at Gamaja (Limpopo Province) in South Africa and in vitro pharmacological evaluation of plants used to manufacture the concoctions. J Evid Based Complem Altern Med. 2017; 22: 805-815. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5871308/
- Wu D, Wang H, Tan J, Wang C, Lin H, et al. Pharmacokinetic and metabolism studies of curculigoside C by UPLC-MS/MS and UPLC-QTOF-MS. Molecules. 2019; 24: 21. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6337338/