Omentin-1 Levels in Hypothyroid Patients with Autoimmune Thyroiditis
By Selin Kir1, Suheyla Gorar2, Isilay Kalan Sari2, Hamit Yasar Ellidag3, Ayhan Hilmi Cekin1, Esin Yilmaz3Affiliations
doi: 10.29271/jcpsp.2023.08.842ABSTRACT
Objective: To determine Omentin-1 in hypothyroid patients with autoimmune thyroiditis compared to controls.
Study Design: Observational study.
Place and Duration of the Study: Department of Internal Medicine and Endocrinology, University of Health Sciences, Antalya Training and Research Hospital, Turkiye, between August 2017 and March 2020.
Methodology: The study included 63 newly diagnosed hypothyroid patients with autoimmune thyroiditis and 40 healthy volunteers. Body mass index, fasting blood glucose, homeostasis model assessment for insulin resistance, lipid profile, thyroid function tests, thyroid autoantibodies, and omentin-1 levels were determined before and after treatment with levothyroxine sodium in all participants.
Results: Omentin-1 was significantly higher in the control subjects [15.05 (12.12-18.06) ng/ml] than in the hypothyroid patients with autoimmune thyroiditis [3.04 (2.39-3.76) ng/ml, p<0.001]. There was no significant difference in omentin-1 level in patients who achieved euthyroidism by treatment (p=0.26). In correlation analysis, serum omentin-1 level was found to correlate negatively with thyroid-stimulating hormone (r=-0.27, p=0.006), anti-thyroid peroxidase (r=-0.32, p=0.001), and anti-thyroglobulin antibodies (r=-0.26, p=0.007), whereas it correlated positively with free triiodothyronine (r=0.22, p=0.021) and free thyroxine (r=0.24, p=0.012).
Conclusion: Lower omentin-1 levels in hypothyroid patients with autoimmune thyroiditis and its negative correlation with thyroid-stimulating hormone suggest that omentin-1 may play some role in hypothyroidism and autoimmune thyroiditis.
Key Words: Hypothyroidism, Chronic autoimmune thyroiditis, Omentin-1.
INTRODUCTION
Hypothyroidism describes a low thyroid hormone level. Overt hypothyroidism is associated with low free thyroxine (FT4) and high thyroid stimulating hormone (TSH) whereas in subclinical hypothyroidism, serum TSH increases, but serum FT4 remains within the normal range. Primary hypothyroidism results from the destruction of the thyroid gland due to different aetiological factors. Chronic autoimmune thyroiditis (AIT) is the most common cause of its iodine-sufficient regions.1,2 Hypothyroidism is an independent risk factor for obesity, dyslipidemia, atherosclerosis, and cardiovascular disease. It is still not clear by which mechanisms and molecules thyroid hormone deficiency affects energy balance, carbohydrate-lipid-protein metabolism, and cardiovascular risk.3-5
Omentin-1, a newly identified adipokine, is released mainly from visceral adipose tissue. Its physiological, pathophysiological, and clinical roles are not yet clearly defined.6 Studies have shown that serum levels of omentin-1 decrease insulin resistance, obesity, metabolic syndrome, arterial stiffness and atherosclerosis.7-9 Moreover, recombinant omentin has been found to enhance glucose uptake and increase insulin induction of Akt/protein kinase B phosphorylation in isolated adipocytes.10 The aim of this study was to investigate the levels of omentin-1 in hypothyroid patients with AIT compared to controls.
METHODOLOGY
This study was conducted at the Department of Internal Medicine and Endocrinology, University of Health Sciences, Antalya Training and Research Hospital, between August 2017 and March 2020. Newly diagnosed hypothyroid patients with no history of acute or chronic systemic disease, thyroid surgery, pregnancy or lactation in the past two years, and not taking those medications that affect omentin-1 levels (angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, thiazides, amiodarone, or lithium, etc.) were included in the study. Total 63 hypothyroid with AIT patients (33 patients with TSH <10 uIU/mL and 30 patients with TSH >10 uIU/mL) who met the criteria recruited in the study. A control group was consisting of 40 healthy volunteers. Informed consent were obtained from all participants.
The diagnosis of AIT was based on the positivity of thyroid antibodies and typical ultrasound findings including pseudonodular appearance and hypoechogenic pattern. TSH ≥5.6 uIU/ml was defined as hypothyroidism due to the upper limit for TSH in using kit ranges. Demographic and examined biochemical data of the study group were recorded. For hypothyroidism therapy, levothyroxine (LT) was given in an individualised, based on each hypothyroid patient’s initial TSH level. And there was no intervention other than LT during the study. Three months after LT treatment, all parameters in the hypothyroid group were repeated. As normalisation of TSH level (0.34-5.6 uIU/ml) could not be achieved in 10 patients and 16 patients did not continue the follow-up, these patients were excluded from the study. Data of 37 patients who completed the study and became euthyroid under LT therapy were recorded. In this prospective observational study, all of the data were compared between groups (hypothyroid with TSH <10 uIU/mL and with TSH >10 uIU/mL and controls). In addition, the pre- and post-treatment data of the hypothyroid patients who completed the LT treatment were analysed.
Body mass index (BMI) of the subjects was determined using Densi automatic bathroom scale GL-150. Fasting blood glucose (FBG), fasting insulin, homeostasis model assessment for insulin resistance (HOMA-IR), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), thyroid hormones, antibodies, and serum omentin-1 were determined of all participants. Blood samples were collected between 08o-10o after 10-12 hours of fasting. FBG, lipids were measured spectrophotometrically (Beckham Coulter AU5800 Diagnostics, Brea, CA). Fasting insulin, TSH, FT3, FT4, anti-TPO, and anti-TG levels were determined by chemiluminescence (Beckham Coulter AccessDxI800 Diagnostics, Brea, CA). The HOMA-IR was calculated using the formula: FBG (mg/dL) x fasting insulin (uIU/mL)/405.11 Blood samples were centrifuged at 4000 rpm for 10 minutes and stored at -80°C for later assay to measure serum omentin-1 level. Omentin-1 level was measured with the Human ITLN1 (Intelectin 1/Omentin) ELISA Kit (BT LAB, Bioassay Technology Laboratory, Shanghai, CHINA) using the enzyme immunoassay sandwich technique. The sensitivity of this ELISA kit is 0.38 ng/ml, the detection range: 0.63-40 ng/ml, within-run coefficient of variation <8%, inter-run coefficient of variation <10% (intra-assay: CV <8%, inter-assay: CV <10%) was determined.
The SPSS 23.0 package program was used for statistical analysis. Normality of the distribution was investigated with Shapiro–Wilks test. When comparing continuous parameters between groups, the independent Student t-test (two groups) and one-way test ANOVA (more than two groups) were used for parametric distribution, and the Mann-Whitney U-test (two groups) and Kruskall Wallis (more than two groups) test were used for nonparametric distribution. Paired t-test (parametric distribution) and Wilcoxon test (nonparametric distribution) were used to compare the results before and after treatment. Correlation analysis was analysed using Pearson and Spearman tests. The p-values less than 0.05 were considered statistically significant for all tests. A post hoc power analysis using the G*Power 3.1 revealed 95% statistical power with 0.05 alpha significance level, and a confidence interval of 95%.
RESULTS
The demographic characteristics and biochemical values of the 63 hypothyroid patients with AIT and the 40 healthy controls are summarised in Table I. TSH, anti-TPO, and anti-TG were significantly increased (p<0.001, p<0.001, p<0.001, respectively), and FT3 and FT4 were significantly decreased (p<0.018, p<0.001, respectively) in patients group. Serum omentin-1 level was significantly lower in hypothyroid patients with AIT than in controls [3.04 (2.39 - 3.76) vs. 15.05 (12.12 - 18.06); p<0.001]. When omentin-1 levels were compared between groups, there was a statistically significant difference in mean omentin-1 levels [3.42 (2.35 - 4.44) ng/mL for TSH <10 uIU/mL, 2.74 (2.11 - 5.12) ng/mL for TSH ≥10 uIU/mL and 15.05 (12.12 - 18.06) ng/mL for controls, p<0.001, Table I, Figure 1].
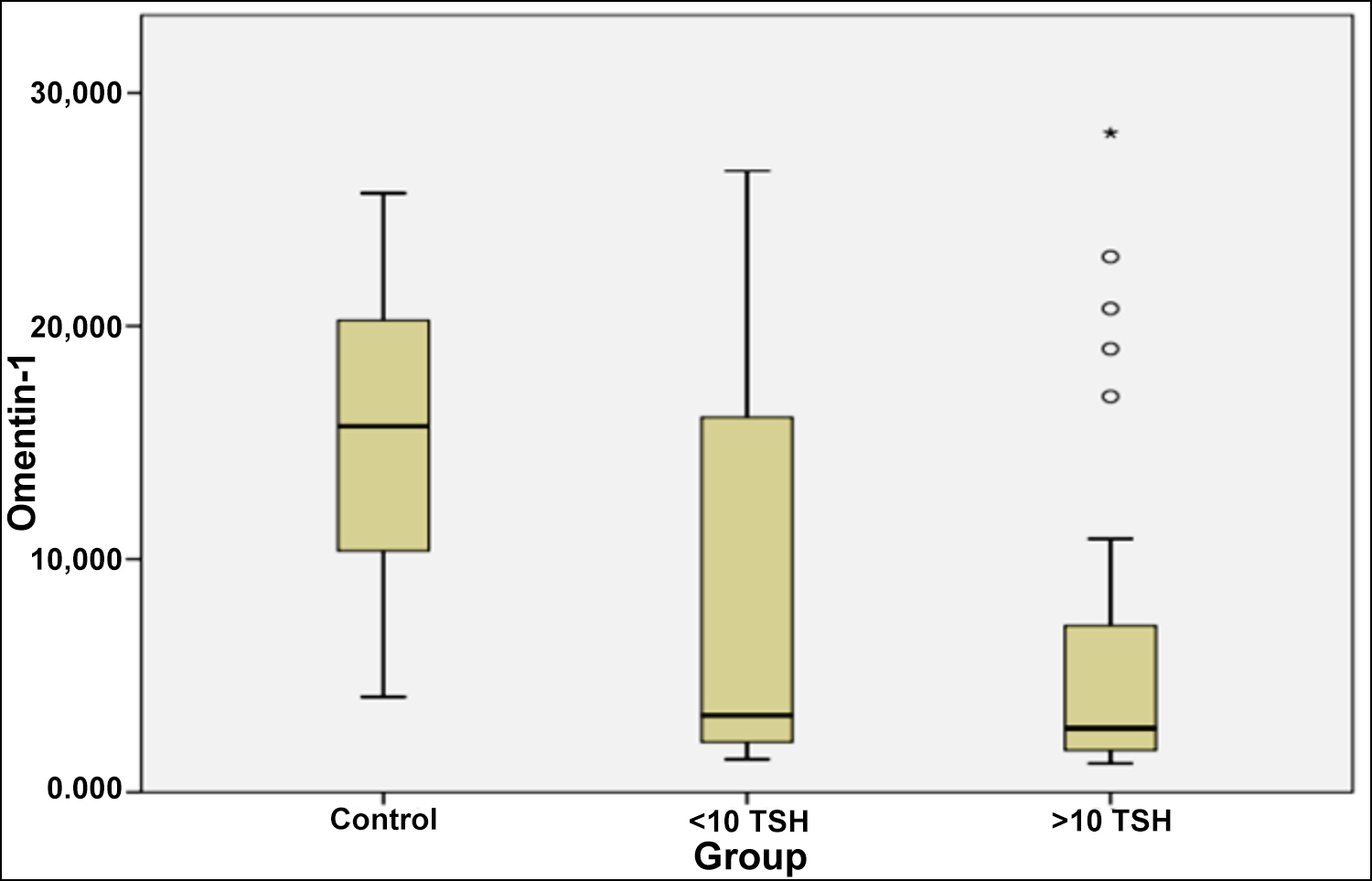 Figure 1: Mean serum omentin-1 level in hypothyroid autoimmune thyroiditis and controls.
Figure 1: Mean serum omentin-1 level in hypothyroid autoimmune thyroiditis and controls.
Data from 37 patients whose TSH levels normalised after treatment with LT were reevaluated. BMI (p = 0.041) was significantly decreased after treatment. Omentin-1 levels decreased slightly during treatment with LT, but this decrease was not statistically significant (p = 0.266, Table II).
Omentin-1 was negatively correlated with TSH (r = -0.272, p=0.006), anti-TPO (r = -0.327, p=0.001), anti-TG (r = -0.266, p=0.007) and positively correlated with FT4 (r = 0.245, p = 0.012), and FT3 (r = 0.228, p=0.021) in correlation analysis (Table III).
DISCUSSION
This study investigated the basal omentin-1 after LT treatment and its relationship with metabolic parameters in hypothyroid patients with AIT.
Table I: Anthropometric, metabolic, and hormonal parameters of hypothyroid patients with autoimmune thyroiditis and healthy controls.
|
|
Hypothyroid AIT |
Control n=40 |
p* |
p† |
||
|
TSH <10 uIU/mL n=33 |
TSH ≥10 uIU/mL n=30 |
All patients n=63 |
||||
|
Age (year) |
43.33±12.03 |
45.0±10.41 |
44.12±11.23 |
40.20±10.76 |
0.082§ |
0.186§ |
|
BMI (kg/m2) |
28.93 (27.13-30.86) |
27.53 (24.67-29.20) |
28.07 (26.99-29.20) |
26.33 (25.20-28.19) |
0.192¶ |
0.186¶ |
|
Fasting glucose (mg/dl) |
90.50 (88.01-95.00) |
88.50 (84.00-95.00) |
90 (88-93) |
91 (85.-97) |
0.906¶ |
0.977¶ |
|
HOMA-IR |
1.86 (1.42-2.92) |
1.31 (0.80-1.95) |
1.63 (1.16-2.10) |
1.46 (1.37-1.61) |
0.499¶ |
0.115¶ |
|
HDL-C (mg/dL) |
48.50 (45.00-52.49) |
51.00 (47.00-56.50) |
50 (47-52) |
55 (51-58) |
0.166¶ |
0.171¶ |
|
LDL-C (mg/dL) |
121.57±37.77 |
137.36±46.87 |
129.09±42.75 |
120.27±28.92 |
0.254§ |
0.134§ |
|
TG (mg/dL) |
109 (82-128) |
119.00 (74-175) |
109 (88-134) |
110 (78-126) |
0.567¶ |
0.579¶ |
|
TSH (uIU/mL) |
7.46 (6.78-8.00) |
19.08 (13.90-32.69) |
9.87 (8.10-11.30) |
1.91 (1.52-2.75) |
<0.001¶ |
<0.001¶ |
|
FT3 (ng/dL) |
3.38±0.37 |
3.08±0.57 |
3.24±0.49 |
3.46±0.34 |
0.018§ |
0.002§ |
|
FT4 (ng/dL) |
0.71 (0.64-0.75) |
0.53 (0.49-0.62) |
0.63 (0.57-0.70) |
0.76 (0.74-0.81) |
<0.001¶ |
<0.001¶ |
|
Anti-TPO (IU/ml) |
277.85 (103.16-338.10) |
426.35 (201.80-755-50) |
325.35(222.70-406.80) |
0.50 (0.35-0.95) |
<0.001¶ |
<0.001¶ |
|
Anti-TG (IU/ml) |
8.35 (3.00-60.20 |
12.50 (4.50-681.30) |
10.30 (4.64-42.60) |
0.30 (0.10-0.80) |
<0.001¶ |
<0.001¶ |
|
Omentin-1 (ng/ml) |
3.42 (2.35-4.44)a,c |
2.74 (2.11-5.12)b,c |
3.04 (2.39-3.76) |
15.05 (12.12-18.06)a,b |
<0.001¶ |
<0.001¶ |
|
TSH: Thyroid-stimulating hormone; FT3: Free triiodothyronine; FT4: Free thyroxine; BMI: Body mass index; FBG: Fasting blood glucose; HOMA-IR: Homeostasis model assessment for insulin resistance index; HDL-C: High-density-lipoprotein cholesterol; LDL-C: Low-density-lipoprotein cholesterol; TG: Triglyceride. |
||||||
Table II: Comparison of parameters and serum omentin-1 levels before and after treatment in the hypothyroid autoimmune thyroiditis.
|
|
Before treatment |
After treatment |
p |
|
Mean ±SD Median (IQR) |
Mean ±SD Median (IQR) |
||
|
BMI (kg/m2) |
28.80 (26.79-29.26) |
28.19 (26.67-29.60) |
0.041* |
|
Fasting glucose (mg/dl) |
89 (85-93) |
91 (89-97) |
0.228 |
|
HOMA-IR |
1.64 (1.00-2.59) |
1.42 (1.07-2.20) |
0.816 |
|
HDL-C (mg/dL) |
52 (50-56) |
52 (50-56) |
0.693 |
|
LDL-C (mg/dL) |
126.60±41.63 |
118.07±32.35 |
0.124 |
|
TG (mg/dL) |
88 (69-128) |
91 (66-141) |
0.801 |
|
TSH (uIU/mL) |
9.52 (7.90-12.89) |
3.74 (2.65-4.32) |
0.001* |
|
FT3(ng/dL) |
3.11±0.45 |
3.34±0.34 |
0.009* |
|
FT4 (ng/dL) |
0.64 (0.55-0.70) |
0.79 (0.76-0.86) |
<0.001* |
|
Omentin-1 (ng/ml) |
3.26 (2.32-5.12) |
2.60 (1.37-7.21) |
0.266 |
|
TSH: Thyroid-stimulating hormone; FT3: Free triiodothyronine; FT4: Free thyroxine; BMI: Body mass index; FBG: Fasting blood glucose; HOMA-IR: Homeostasis model assessment for insulin resistance index; HDL-C: High-density-lipoprotein cholesterol; LDL-C: Low-density-lipoprotein cholesterol; TG: Triglyceride. Note: Values are expressed as mean +SD and median (interquartile range). Paired t-test and Wilcoxon test were used. |
|||
Table III: Correlation analyses between serum omentin-1 levels and other parameters (n: 103).
|
|
Serum omentin-1 |
|
|
r |
p |
|
|
BMI (kg/m2) |
-0.013 |
0.979 |
|
Fasting glucose (mg/dl) |
0.105 |
0.291 |
|
HOMA-IR |
-0.055 |
0.584 |
|
TSH (uIU/mL) |
-0.272 |
0.006** |
|
FT3 (ng/dL) |
0.228 |
0.021* |
|
FT4 (ng/dL) |
0.245 |
0.012* |
|
HDL-C (mg/dL) |
-0.024 |
0.808 |
|
LDL-C (mg/dL) |
-0.027 |
0.788 |
|
TG (mg/dL) |
0.060 |
0.544 |
|
Anti-TPO (IU/ml) |
-0.327 |
0.001** |
|
Anti-TG (IU/ml) |
-0.266 |
0.007** |
|
TSH: Thyroid-stimulating hormone; FT3: Free triiodothyronine; FT4: Free thyroxine; BMI: Body mass index; FBG: Fasting blood glucose; HOMA-IR: Homeostasis model assessment for insulin resistance index; HDL-C: High-density-lipoprotein cholesterol; LDL-C: Low-density-lipoprotein cholesterol; TG: Triglyceride. |
||
There are few studies in the literature on omentin-1 in thyroid dysfunction. Cerit et al. studied serum omentin-1 levels in 28 cases with overt hypothyroidism with AIT. Omentin-1 levels were significantly decreased in patients with hypothyroidism compared with controls. They observed a significant increase in omentin-1 level along with a significant decrease in weight, BMI, LDL-C, HDL-C, lean body mass, and epicardial adipose tissue thickness in the hypothyroid group after achieving euthyroidism with LT. In addition, negative correlations were found between omentin-1 and BMI, lean body mass, LDL-C, TG, and TSH. There was a positive correlation between omentin-1 and FT4 and FT3. In interpreting the study results, the authors reported that increased epicardial adipose tissue thickness and low omentin-1 levels may be potential risk factors in hypothyroidism-associated atherosclerosis.12
Another study conducted by Gao et al. that thyroid hormones and omentin-1 were evaluated in 240 newly diagnosed cases of subclinical hypothyroidism. Omentin-1 levels were highest in the control group, lower in patients with a TSH <10 mIU/l, and lowest in patients with a TSH>10 mIU/l. Euthyroidism was achieved after treatment with LT, and a significant increase in omentin-1 levels and a significant decrease in LDL levels were observed. There was a negative correlation between omentin-1 and TSH. The authors indicated that omentin-1 may protect endothelial functions in the pathogenesis of atherosclerosis in subclinical hypothyroidism.13
The levels of omentin-1 were examined in 70 euthyroid and 70 hyperthyroid patients by Alshaikh et al. It was found that omentin-1 levels were significantly lower in hyperthyroid patients than in euthyroid patients.14 In this study, the low omentin-1 levels in hypothyroid patients and the negative correlation between TSH and omentin-1 are consistent with the data of other studies performed in hypothyroid patients. However, the authors have not observed any significant improvement in omentin-1 levels by treatment with LT, which differed from one of the points stated by other studies. The aforementioned studies show low omentin-1 levels in hyperthyroidism, similar to results in hypothyroidism which creates a relationship that needs explanation.
The possible relationship between hypothyroidism and its treatment and BMI and insulin resistance still remains unclear. This data also supports that BMI and HOMA-IR values were similar between the hypothyroid and control groups after and before treatment. In addition, there was no correlation between BMI and HOMA-IR with omentin-1. In some studies in which euthyroidism was achieved with LT treatment in hypothyroid patients, it was shown that despite the reduction in weight and BMI, fat and bone mass remained almost unchanged.12,15 Although no lifestyle changes and no specific diet were recommended in this study, there was a significant decrease in weight and BMI after treatment, similar to the studies mentioned above. The results showed that omentin-1 levels did not increase as expected despite the decrease in patients' weight and BMI, thus it was interpreted that patients' weight loss may be due to a decrease in lean mass rather than adipose tissue.
Adipose tissue contains not only adipocytes, but also many T and myeloid cells, which are associated with inflammatory states.16 Especially, obesity is a proinflammatory phenotype which increases the number of immune cells. In this regard, it is not surprising that some inflammatory immune diseases are related with obesity and some adipokines, including one newly defined omentin-1.16 There is a negative correlation between gene expression and circulating levels of omentin-1 and obesity.7 Omentin-1 exerts its anti-inflammatory effects by suppressing JNK activation via the AMPK/eNOS pathway and inhibits monocyte adhesion by inhibiting both the ERK /NFκB and p38/JNK pathways.17,18 Cerit et al. also found low omentin-1 levels in patients with autoimmune hypothyroidism who had similar characteristics to this study.12 A negative correlation was found between thyroid autoantibodies and omentin-1 levels in patients, also.
Omentin-1 levels are associated with atherosclerosis and various cardiovascular diseases, as the significant decrease in omentin-1 levels has been found in those situations.12,13,18 Du et al. have reported that TG and omentin-1 are related independently of coronary artery disease.19 In the study on omentin-1 level in acute and stable coronary syndrome, it was reported that omentin-1 and HDL-C were significantly higher, LDL-C was significantly lower in the healthy controls. However, no correlation was found between omentin-1 and lipid parameters.20 In this study, although omentin-1 levels were significantly higher in controls than in patients, there was no significant difference and correlation between omentin-1 and lipids. However, after treatment, there was a decrease in LDL-C and an increase in HDL-C, but this was not statistically significant. Based on this result, the authors hypothesised that omentin-1 and HDL-C levels tend to decrease in hypothyroidism.
This study has some limitations. First, the number of patients who completed treatment was small. Three months may be too short time to measure omentin-1 levels, and the time of metabolic recovery after euthyroidism could be longer. Body fat and muscle distribution analysis could be performed. Other obesity-related adipokines and cardiovascular risk factors could be studied with omentin-1.
CONCLUSION
Serum omentin-1 levels were lower in hypothyroid patients with AIT, and its negative correlation with thyroid-stimulating hormone. Large-scale clinical studies are needed to eventually evaluate whether omentin-1 may play a role in hypothyroidism and AIT.
ETHICAL APPROVAL:
This study was approved by the Ethics Committee of the Antalya Training and Research Hospital (Date: 30/11/2017, Number: 18/02) and was conducted in accordance with the government policies and the Declaration of Helsinki.
PATIENTS’ CONSENT:
Written informed consent were obtained from all the patients.
COMPETING INTEREST:
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTION:
SK: Data curation and literature review.
SG: Conceptualisation, data curation, project administration, methodology, and literature review.
IKS: Writing the original draft.
HYE: Biochemical analysis.
AHC: Formal analysis.
EY: Visualisation.
All the authors have approved the final version of the manu-script.
REFERENCES
- Nygaard B. Hypothyroidism (primary). BMJ Clin Evid 2010; 2010:0605.
- Tunbridge WM, Evered DC, Hall R, Appleton D, Brewis M, Clark F, et al. The spectrum of thyroid disease in a community: The Whickham survey. Clin Endocrinol (Oxf) 1977; 7(6):481-93. doi: 10.1111/j.1365-2265.1977.tb01340.x.
- Knapp M, Lisowska A, Sobkowicz B, Tycinska A, Sawicki R, Musial WJ. Myocardial perfusion and intima-media thickness in patients with subclinical hypothyroidism. Adv Med Sci 2013; 58(1):44-9. doi: 10.2478/v10039-012-0068-9.
- Cabral MD, Teixeira P, Soares D, Leite S, Sallee E, Waisman M. Effects of thyroxine replacement on endothelial function and carotid artery intima-media thickness in female patients with mild subclinical hypothyroidism. Clinics (Sao Paulo) 2011; 66(8):1321-8. doi: 10.1590/s1807-5932 2011000800003.
- Sanyal D, Raychaudhuri M. Hypothyroidism and obesity: An intriguing link. Indian J Endocrinol Metab 2016; 20(4): 554-7. doi: 10.4103/2230-8210.183454.
- Zengi S, Zengi O, Kirankaya A, Kucuk H, Kutanis EE, Yiğit O. Serum omentin-1 levels in obese children. J Pediatr Endocrinol Metab 2019; 32(3):247-51. doi: 10.1515/ jpem-2018-0231.
- de Souza Batista CM, Yang RZ, Lee MJ, Glynn NM, Yu DZ, Pray J, et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes 2007; 56(6):1655-61. doi: 10.2337/db06-1506.
- Jialal I, Devaraj S, Kaur H, Adams-Huet B, Bremer AA. Increased chemerin and decreased omentin-1 in both adipose tissue and plasma in nascent metabolic syndrome. J Clin Endocrinol Metab 2013; 98(3):514-7. doi: 10.1210/ jc.2012-3673.
- Yoo HJ, Hwang SY, Hong HC, Choi HY, Yang SJ, Seo JA, et al. Association of circulating omentin-1 level with arterial stiffness and carotid plaque in type 2 diabetes. Cardiovasc Diabetol 2011; 10:103. doi: 10.1186/1475-2840- 10-103.
- Pan Guo L, Li Q. Changes of serum omentin-1 levels in normal subjects and in patients with impaired glucose regulation and with newly diagnosed and untreated type 2 diabetes. Diabetes Res Clin Pract 2010; 88(1):29-33. doi: 10.1016/j.diabres.2010.01.013.
- Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European group for the study of ınsulin resistance (EGIR). Diabet Med 1999; 16(5):442-3. doi: 10.1046/j.1464-5491.1999.00059.x.
- Cerit ET, Akturk M, Altinova AE, Tavil Y, Ozkan C, Yayla C, et al. Evaluation of body composition changes, epicardial adipose tissue, and serum omentin-1 levels in overt hypothyroidism. Endocrine 2015; 49(1):196-203. doi: 10.1007/ s12020-014-0460-2.
- Gao CX, Yang B, Guo Q, Wei LH, Tian LM. High thyroid-stimulating hormone level is associated with the risk of developing atherosclerosis in subclinical hypothyroidism. Horm Metab Res 2015; 47(3):220-4. doi: 10.1055/s-0034- 1394370.
- Alshaikh EM, Omar UM, Alsufiani HM, Mansouri RA, Tarbiah NI, Alshaikh AA, et al. The potential influence of hyperthyroidism on circulating adipokines chemerin, visfatin, and omentin. Int J Health Sci (Qassim) 2019; 13(2): 44-7.
- Karmisholt J, Andersen S, Laurberg P. Weight loss after therapy of hypothyroidism is mainly caused by excretion of excess body water associated with myxoedema. J Clin Endocrinol Metab 2011; 96(1):99-103. doi: 10.1210/jc. 2010-1521
- Hutcheson J. Adipokines influence the inflammatory balance in autoimmunity. Cytokine 2015; 75(2):272-9. doi: 10.1016/ j.cyto.2015.04.004.
- Watanabe T, Watanabe-Kominato K, Takahashi Y, Kojima M, Watanabe R. Adipose tissue-derived omentin-1 function and regulation. Compr Physiol 2017; 7(3):765-81. doi: 10.1002/cphy.c160043.
- Zhou Y, Zhang B, Hao C, Huang X, Li X, Huang Y, et al. Omentin-A novel adipokine in respiratory diseases. Int J Mol Sci 2017; 19(1):73. doi: 10.3390/ijms19010073.
- Du Y, Ji Q, Cai L, Huang F, Lai Y, Liu Y, et al. Association between omentin-1 expression in human epicardial adipose tissue and coronary atherosclerosis. Cardiovasc Diabetol 2016; 15:90. doi: 10.1186/s12933-016-0406-5.
- Zhong X, Zhang HY, Tan H, Zhou Y, Liu F, Chen F, et al. Association of serum omentin-1 levels with coronary artery disease. Acta Pharmacol Sin 2011; 32(7):873-8. doi: 10.1038/aps.2011.26.