Idiopathic Venous Thromboembolism and Metabolic Syndrome: A Meta-analysis
By Refukaiti Abuduhalike, Umesh Yadav, Juan Sun, Ailiman MahemutiAffiliations
doi: 10.29271/jcpsp.2022.07.909ABSTRACT
Metabolic syndrome (MetS) is a recognised risk factor for arterial thromboembolism. However, whether MetS is also a risk factor for venous thromboembolism (VTE) is uncertain. PubMed, Embase, Web of science, and Cochrane databases were searched for case-control and cohort studies as well as conference proceedings of the International society on Thrombosis and Haemostasis (ISTH), and the Women's Health International Symposium Thrombosis and Hemostasis Branch (WHITH) published on or before March 1, 2021, to identify eligible studies. All included articles were assessed by two investigators using the Newcastle–Ottawa scale (NOS). We calculated odds ratios (ORs) and 95% confidence intervals (CIs) to evaluate the association between VTE and MetS by using random or fixed-effects models. There were 31 case-control and 5 cohort studies with a total of 78,529 participants that fulfilled the inclusion criteria, MetS (OR 1.49; 95% CI 1.29–1.73) and its critical component obesity (OR 2.03; 95% CI 1.74–2.37), hypertension (OR 1.40; 95% CI 1.19–1.64) and diabetes mellitus (OR 1.22; 95% CI 1.01–1.48) were significant risk factors for VTE. MetS and its critical component obesity may contribute to the multifactorial pathogenesis of VTE.
Key Words: Venous thromboembolism, Metabolic syndrome, Obesity.
INTRODUCTION
Venous thromboembolism (VTE) is the third leading cause of cardiovascular death in the world besides myocardial infarction and stroke.1,2 Surgery, pregnancy, hormone therapy, cancer, and trauma are the acquired risk factors of VTE.3 As well as interventions for various risk factors, have not decreased the incidence of VTE, and the cause of 30% of the patients with VTE is still unknown.4 Therefore, further exploration of the etiology and pathogenesis of idiopathic VTE, and seeking more effective new ideas for the prevention and treatment of idiopathic VTE is urgent.
Metabolic syndrome (MetS) is a general term for a class of risk factors related to cardiovascular metabolism.5 Recent related research demonstrated that the arterial and venous thromboembolic diseases may be different manifestations of the same chronic non-specific inflammatory diseases.6-8 At the same time, a large number of studies have confirmed the MetS and its critical component, obesity, may affect all the components of Virchow's triangle (coagulation disorders, slowed blood flow, and endothelial damage).9-11
As the co-mediating factors of inflammation and coagulation disorder, MetS and obesity may be considered as the primary risk factors for thromboembolic disease. However, the association between idiopathic VTE and MetS and its components has not been fully established. This meta-analysis was conducted to determine the risk of idiopathic VTE in MetS.
METHODOLOGY
This research followed the PRISMA statement of systematic review and meta-analysis. No Ethics Committee approval was necessary for meta-analysis.
PubMed, Embase, Web of Science, Cochrane databases and conference proceedings of the International Society on Thrombosis and Haemostasis (ISTH), Women's Health International Symposium Thrombosis and Haemostasis Branch (WHITH), were searched by using the key words: venous thromboembolism or pulmonary thromboembolism or deep vein thrombosis and metabolic syndrome or X syndrome or obesity or body mass index or hypertension or diabetes mellitus or triglycerides.
Studies were included if they were case-control or cohort studies on the patients with idiopathic VTE and MetS or components of MetS, especially obesity published on or before March 1 2021. Studies that were duplicate reports of reviews, cross-sectional studies, case reports or studies with no comparable data were excluded.
Study selection was independently performed by the two reviewers (Abuduhalike and Yadav) with disagreements resolved through discussion and by seeking the opinion of a third reviewer (Sun), whenever needed to reach the consensus. Only studies reporting on objectively confirmed diagnosis of idiopathic VTE, were included in the final data set.1
Information regarding the year of publication, study design, the country where the research subjects are located, the sample size and the control group, the definition of VTE used and MetS, the adequacy of exposure, and the length of follow-up years were retrieved about all the selected studies. All the included articles were assessed by the two investigators (Abuduhalike and Yadav) using the NOS scale.12 The NOS scale comprises a total of 8 items, with a full score of 9 points. All the studies with a NOS score of ≥6 are considered high-quality studies, with relatively reliable results. All the authors discussed together before deciding the NOS score for each selected study.
The results of the studies were collected using Review Manager (RevMan), version 5.3 for the Windows (The Cochrane Collaboration 2003, Oxford, England). The authors calculated odds ratios (ORs) and 95% confidence intervals (CIs) to estimate the risk for each factor by using the random or fixed-effects model. Statistical heterogeneity was evaluated with χ2 and I2 statistics, which assess the appropriateness of collecting the individual study results.13 For I2 ≥50%, a random-effects model was used, otherwise, a fixed-effects model was used in accordance with the Cochrane review guidelines. Publication bias was evaluated using funnel plots.
RESULTS
Seven thousand five hundred and ninety studies were identified using the search strategy, out of which 7,511 were excluded after scanning the titles and abstracts. In order to avoid the influence of confounding factors on the results of this meta-analysis, surgery, pregnancy, and cancer related VTE were not included in this study. After excluding irrelevant articles based on the process illustrated in Figure 1, 31 case-control and 5 cohort studies with a total of 78,529 participants were eventually included in the meta-analysis. NOS score was determined for all the selected studies. All the studies with an NOS score of ≥6 were considered high-quality studies, and the results were relatively reliable.
As shown in Figure 2, 5 case-control and 2 cohort studies evaluated the association between VTE and MetS.14-20 After a general assessment of the OR values of all the included studies, it can be deduced that the individuals with MetS have a higher risk of VTE than those without MetS (OR 1.49; 95% CI 1.29–1.73). The heterogeneity among the 5 case-control studies and 2 cohort studies included in this analysis was extremely low (I2 = 0%). There was a moderate degree of heterogeneity between the case-control and cohort studies (I2 = 41%). As only 5 case-control studies and 2 cohort studies were included, publication bias analysis was not required.
As shown in Figure 3, 17 case-control and 3 cohort studies evaluated the effect of obesity on VTE. 14,16-18,21-36 Body mass index ≥30 Kg/m2 was grouped together and defined as obesity. As can be seen from the forest plot, whether in case-control or cohort studies, obese subjects have a higher risk of developing VTE than non-obese subjects (OR 2.03; 95% CI 1.74–2.37).
There was high heterogeneity among the 17 case-control studies included (I2=64). Heterogeneity could not be significantly reduced after subgroup analysis based on the quality of the included studies, the year of publication, the total number of subjects included, and the source of the study population. Finally, the source of heterogeneity was analysed by the stepwise elimination method, and it was determined that the heterogeneity obviously decreased after elimination of the study published by Hotoleanu et al. (I2=56). A moderate degree of heterogeneity (I2=58%) was found between the case-control studies and cohort studies included in this section, therefore, it is believed that the heterogeneity originates from the large number of case-control studies and the comprehensive effect of the quality, included subject numbers, and the inclusion and exclusion criteria among the included studies.
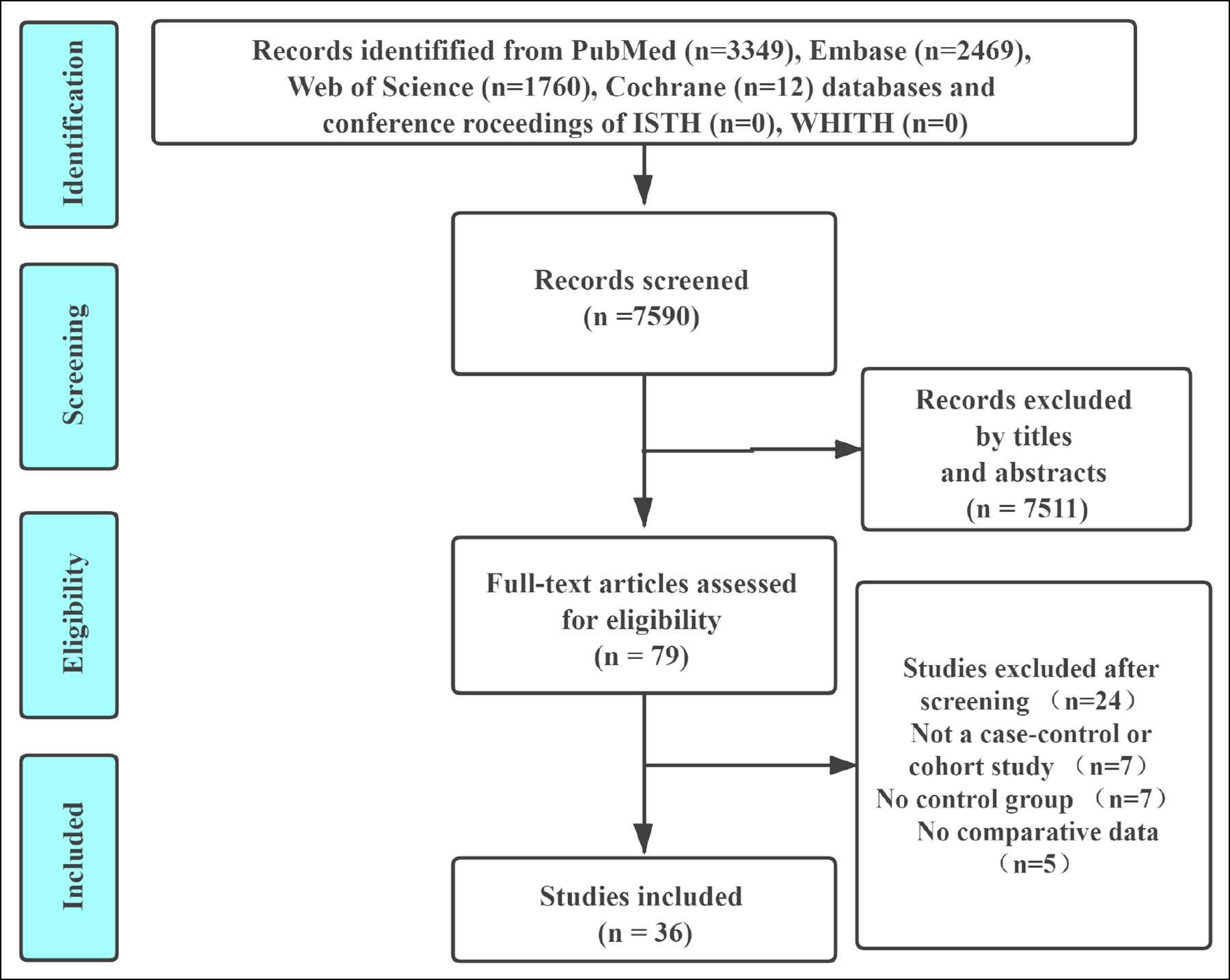 Figure 1: The process of article selection.
Figure 1: The process of article selection.
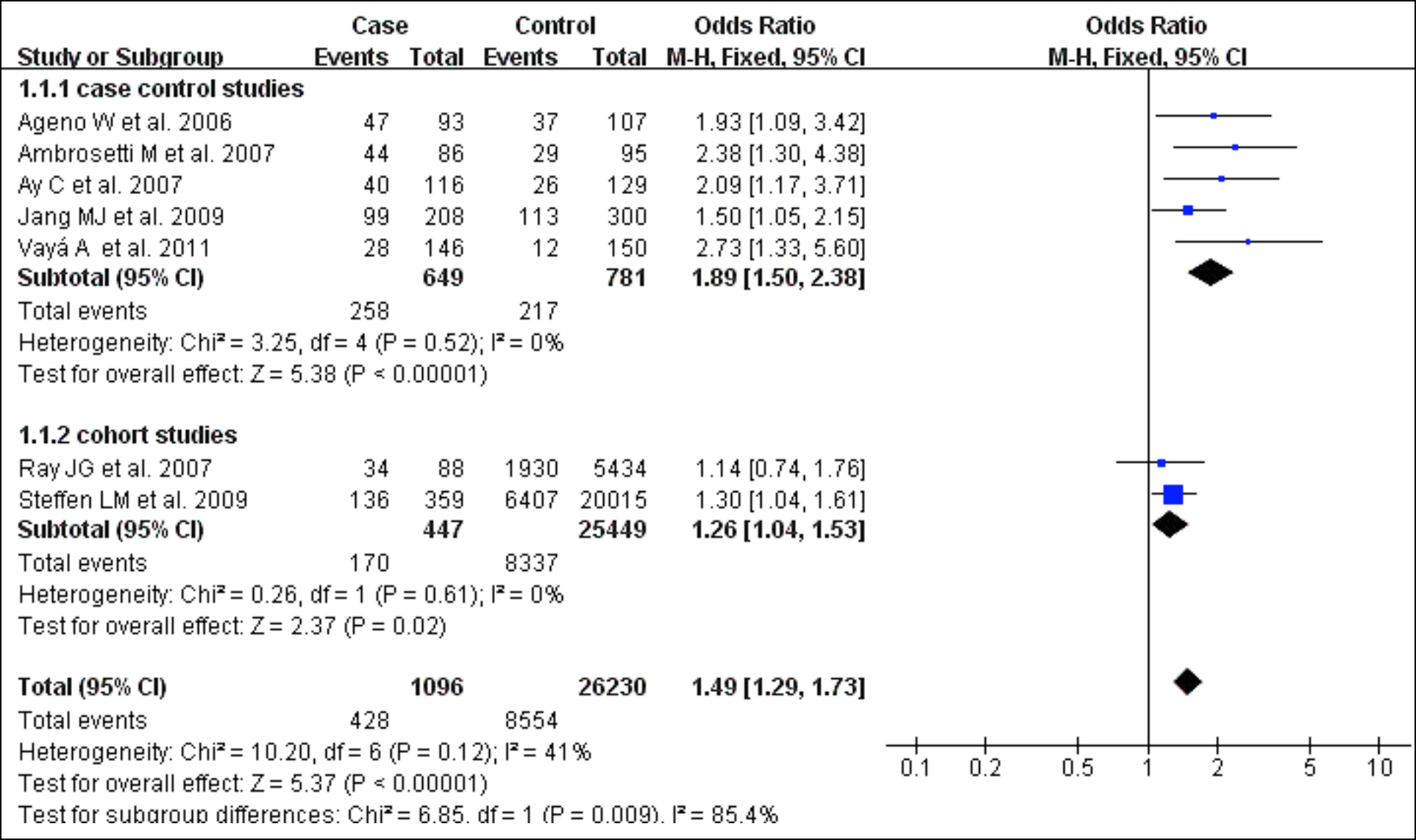 Figure 2: Forest plot of the relationship between VTE and MetS.
Figure 2: Forest plot of the relationship between VTE and MetS.
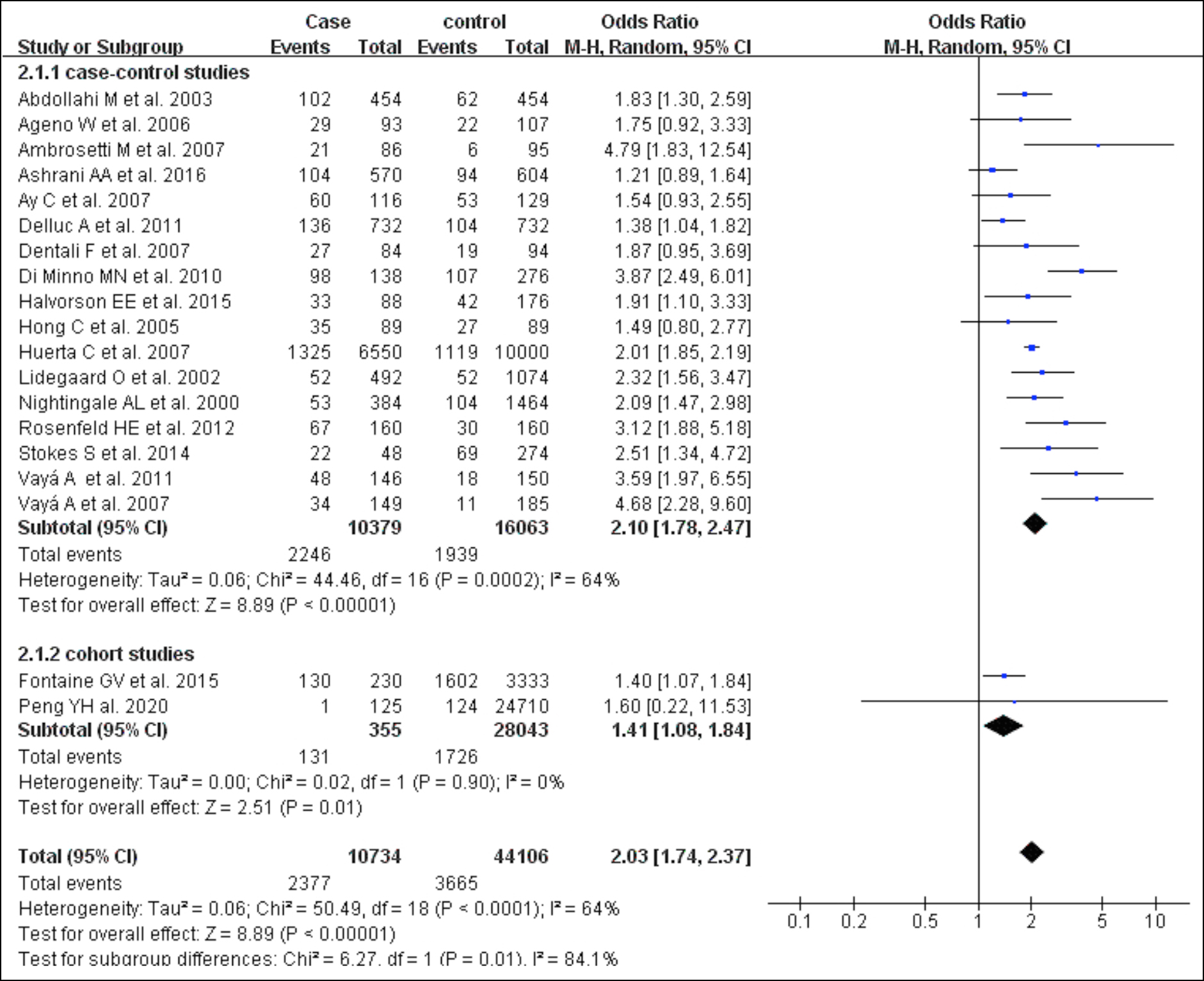 Figure 3: Forest plot of the relationship between VTE and obesity.
Figure 3: Forest plot of the relationship between VTE and obesity.
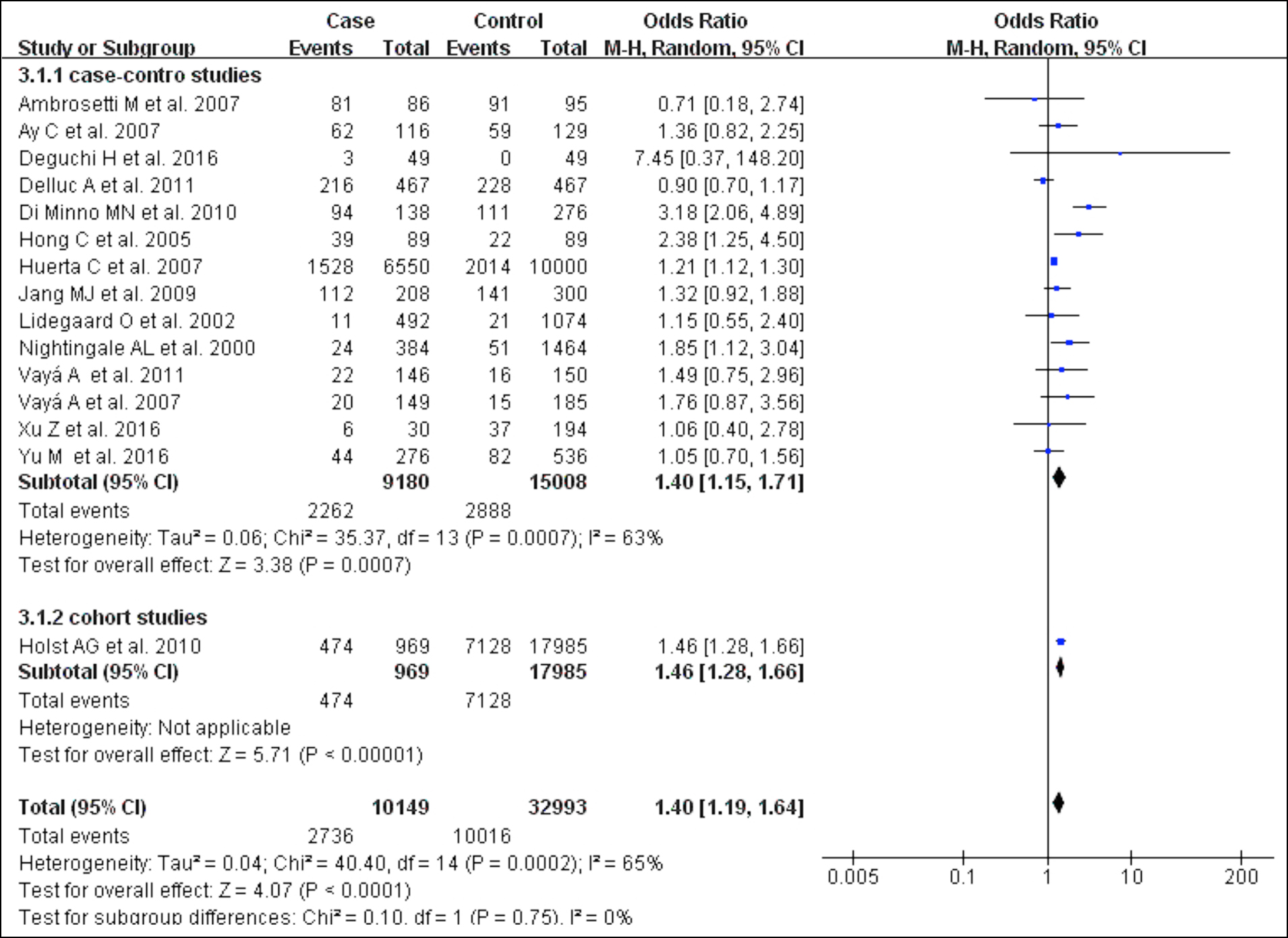 Figure 4: Funnel plot of the relationship between VTE and hypertension.
Figure 4: Funnel plot of the relationship between VTE and hypertension.
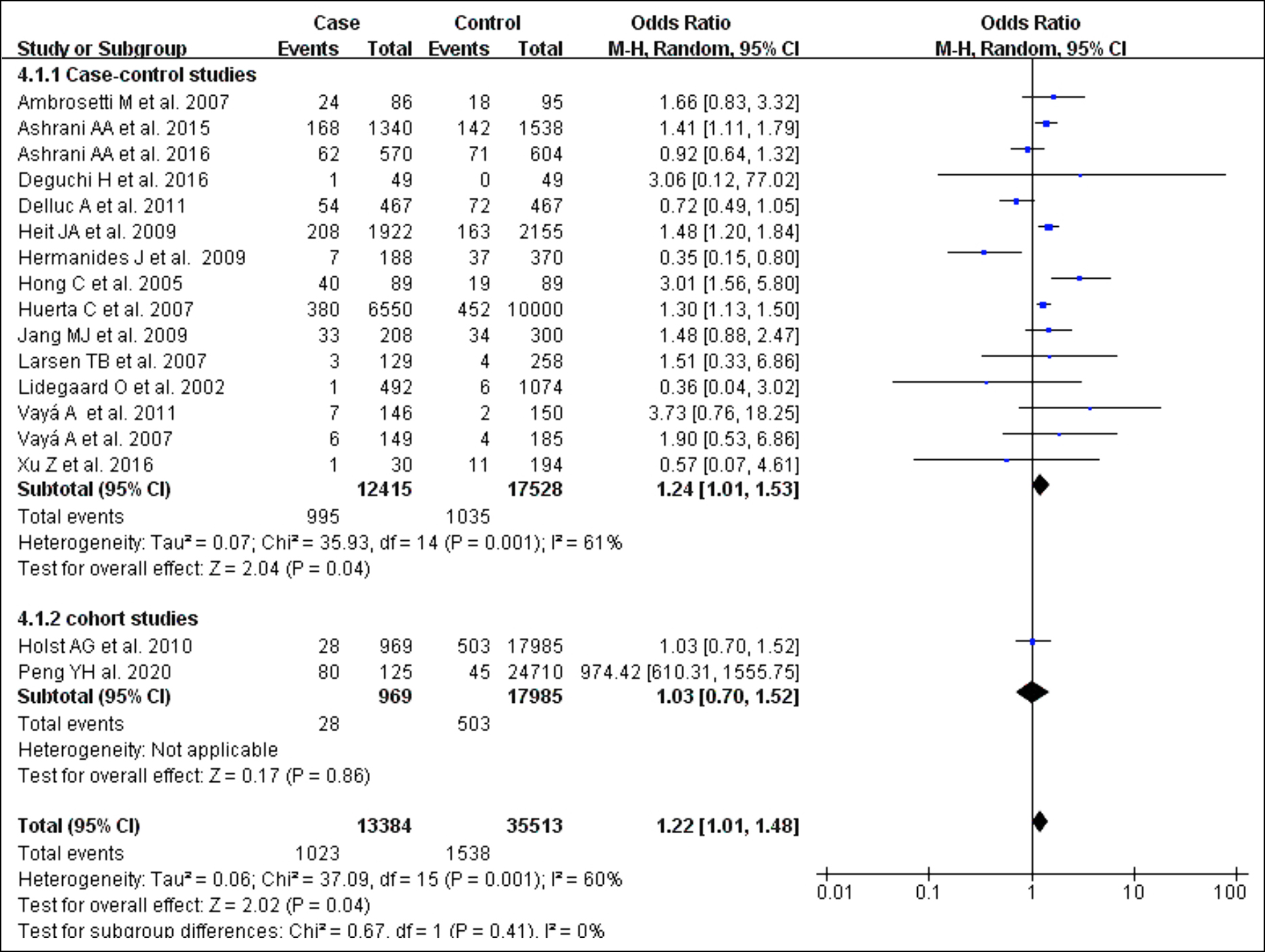 Figure 5: Forest plot of the relationship between VTE and diabetes mellitus.
Figure 5: Forest plot of the relationship between VTE and diabetes mellitus.
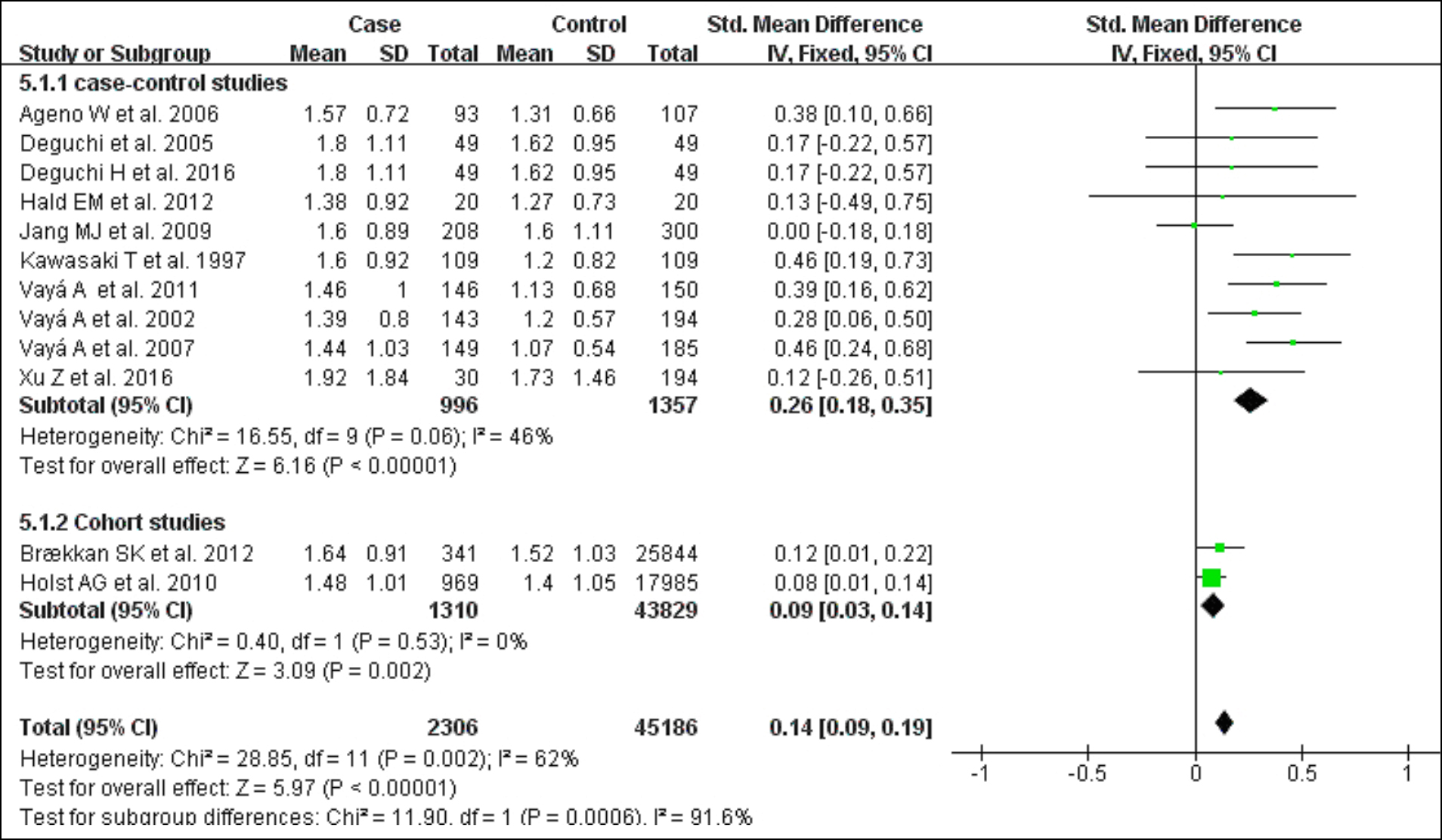 Figure 6: Forest plot of the relationship between VTE and triglycerides.
Figure 6: Forest plot of the relationship between VTE and triglycerides.
In order to avoid the high publication bias, the authors enlarged their search terms, identified a large number of studies in the multiple databases and conducted detailed reviews. Therefore, this section includes a large number of studies published from 2000 to 2020. The funnel plot reveals that moderate publication bias exists among the included studies. It is believed that there are many sources of publication bias and the difficulties of the publication of research papers with negative statistical results, is a common problem worldwide. Consequently, the heterogeneity and publication bias could have affected results to a certain extent.
As shown in Figure 4, 14 case-control studies examined the association between hypertension and VTE.1,17,19,21,23-26,29-34 Patients with hypertension (OR 1.40; 95% CI 1.19–1.64) were at higher risk of developing VTE, with statistical heterogeneity among the studies (I2=65%, p<0.001). After a subgroup analysis by stepwise elimination, it was determined that the heterogeneity was significantly improved after removing the article of Di Minno et al. (I2=27%).24
As shown in Figure 5, 15 case-control studies investigated to examine the association between diabetes mellitus and VTE.17-19,23-25,29-33,35-38 Patients with Diabetes mellitus were at higher risk of VTE (OR 1.22; 95% CI 1.01–1.48) with statistical heterogeneity among the studies (I2=60%; p=0.001). In order to find the source of heterogeneity, the selected papers were divided into three groups for subgroup analysis based on the total number of subjects (n≥1000, 500≤n<1000, n<500). The results of the subgroup analysis suggest that the heterogeneity mainly originates from the papers with a total number of subjects from 500 to 1000.
As shown in Figure 6,10 case-control studies evaluated the association between triglyceride levels and risk of VTE.16,29-33,39-42 The level of measured triglycerides was higher in patients with VTE than in controls (Standardised Mean Difference (SMD) 0.14; 95% CI 0.09–0.19) with medium statistical heterogeneity among the studies (I2 =62%; p=0.002).
DISCUSSION
The association between MetS and VTE has been studied for nearly 20 years, but no clear consensus has been achieved and the underlying mechanisms have not been elucidated. In clinical practice, the authors emphasise the importance of intervention for MetS in the patients with arterial thromboembolic diseases, but when the patients at higher risk of developing idiopathic VTE are encountered, the importance of intervention for MetS is often not stressed. The results suggest that MetS (OR 1.49; 95% CI 1.29–1.73) is the significant risk factor for VTE, and further confirmed the importance of early intervention for MetS to prevent idiopathic VTE. In addition, the NOS evaluation of the included studies is shown as high-quality (NOS Scale ≥6) with extremely low heterogeneity (I2 = 0), thereby demonstrating the reliability of our results.
Obesity is a serious, global health problem. The latest report of the World Health Organization (WHO) revealed that in 2016, 1.9 billion of the world’s adults were overweight and more than 650 million were obese.37 Since, the exact amount of risk that obesity contributes in idiopathic VTE has not been ascertained, the results clearly suggest that obesity (OR 2.03; 95% CI 1.74–2.37) is the main risk factor in idiopathic VTE. There is high heterogeneity (I2=64) in this part of the study and the heterogeneity obviously decreased after the elimination of the study published by Hotoleanu et al. (I2=56). The funnel plot results reveal that there is publication bias among the included studies. Therefore, it is believed that there are many sources of publication bias and the difficulties of the publication of research papers with negative statistical results, is a common problem worldwide.
The findings of this meta-analysis reveal that some components of the MetS, namely hypertension (OR 1.40), and diabetes mellitus (OR 1.22) are significantly associated with the risk of VTE. In addition, triglycerides levels were significantly higher in VTE patients with MetS compared to controls, an established fact, but without significant association with the risk of VTE. However, the analysis is unable to determine whether MetS is a stronger risk factor than each of these three components in isolation. Nevertheless, these findings could indicate that MetS may be one element of the multifactorial pathogenesis of VTE. However, although an association exists between MetS and some of its components with the risk of VTE, a direct causality link is difficult to establish.
In order to reveal the intrinsic pathophysiological relationship between MetS and idiopathic VTE, the authors consulted a large number of studies and found that the potential causes of MetS and obesity increasing the risk of VTE may be related to the factors such as increased coagulation activity, vascular endothelial injury, venous blood stasis, oxidative stress, and chronic inflammation.10,11,38-46 At the same time, coagulation disorder, slowed blood flow, and endothelial damage are the key components of Virchow,s triangle.9,47-49 In addition, adipose tissue can also secrete leptin, which up-regulates the expression of tissue factors, thereby, promoting the pre-thrombotic state.43,44 The mechanism of idiopathic VTE caused by MetS and its components are more complicated, but eventually lead to idiopathic VTE after causing the lesions of various components of Virchow's triangle. The above research results support the results of findings and further reveal the pathophysiological mechanism of idiopathic VTE caused by MetS. Finally, weight loss and exercise prescription may reduce the risk of VTE associated with metabolic syndrome and obesity, which cannot be ignored in our clinical practice, particularly in the later stage.39,50
There are several potential limitations in this study. First, there is some degree of heterogeneity in the included studies, which could indicate differences in the population demographics, sample size, patient characteristics, and exposure misclassification due to variation in the accuracy of monitoring, which decreases the scientific validity of the meta-analysis and its conclusions. Second, the included studies have been conducted over different time periods (2000–2021), and it is unclear how changing clinical practices and the introduction and uptake of new diagnostic and therapeutic approaches could have influenced referral, treatment, and diagnostic patterns. Another interesting aspect of this study is that the risk of obesity for idiopathic VTE (OR 2.03) is higher than the risk of MetS (OR 1.49). The reason may be related to the included studies on the association between MetS and VTE, which may be relevant to the small number of studies, small sample size, major retrospective studies, and the impact of merging heterogeneous data on the results. In addition, the asymmetry of funnel plots is a common defect of the most meta-analyses. A possible reason for the asymmetry of the funnel plot is that the works with positive results are more easily accepted by journals, whereas research with negative results, is less likely to be published by journals.
CONCLUSION
MetS (OR 1.49; 95% CI 1.29–1.73) and its critical components: obesity (OR 2.03; 95% CI 1.74–2.37), hypertension (OR 1.40; 95% CI 1.19–1.64) and diabetes mellitus (OR 1.22; 95% CI 1.01–1.48), significantly increase the risk of idiopathic VTE. In future clinical practice, clinicians need to be more attentive to the danger of MetS in the high-risk idiopathic VTE patients and focus on the role of weight loss prescriptions on the prevention of idiopathic VTE. On the basis of this meta-analysis, further multi-centred, prospective, cohort studies are required to further confirm the impact of MetS intervention on primary prevention of idiopathic VTE.
FUNDING INFORMATION:
This work was supported financially by the National Natural Science Fund of China (81560803).
COMPETING INTEREST:
The authors declare no competing interests.
AUTHORS’ CONTRIBUTION:
AM: Conceptualisation, supervision, writing review and editing.
RA, UY: Data curation, investigation.
RA, UY, JS: Formal analysis.
JS: Software.
RA: Writing original draft.
All authors approved the final version of the manuscript to be published.
REFERENCES
- Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GN, Harjola VP, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European respiratory society (ERS). Eur Heart J 2020; 41(4):543-603. doi: 10.1093/eurheartj/ ehz405.
- Di Nisio M, van Es N, Buller HR. Deep vein thrombosis and pulmonary embolism. Lancet 2016; 388(10063):3060-73. doi: 10.1016/S0140-6736(16)30514-1.
- Nicholson M, Chan N, Bhagirath V, Ginsberg J. Prevention of venous thromboembolism in 2020 and beyond. J Clin Med 2020; 9(8)-2467. doi: 10.3390/jcm9082467.
- Heit JA, O'Fallon WM, Petterson TM, Lohse CM, Silverstein MD, Mohr DN, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: A population-based study. Arch Intern Med 2002; 162(11):1245-8. doi: 10.1001/archinte.162.11.1245.
- Alberti KG, Zimmet P, Shaw J. The metabolic syndrome - a new worldwide definition. Lancet 2005; 366(9491): 1059-62. doi: 10.1016/S0140-6736(05)67402-8.
- Delluc A, Lacut K, Rodger MA. Arterial and venous thrombosis: What's the link? A narrative review. Thromb Res 2020; 191:97-102. doi: 10.1016/j.thromres.2020. 04.035.
- Prandoni P. Venous and arterial thrombosis: Two aspects of the same disease? Eur J Intern Med 2009; 20(6):660-1. doi: 10.1016/j.thromres.2020.04.035.
- Abuduhalike R, Sun J, Zhao L, Mahemuti A. Correlation study of venous thromboembolism with SAA, IL-1, and TNF-a levels and gene polymorphisms in Chinese population. J Thoracic Dis 2019; 11(12):5527-34. doi: 10.21037/jtd. 2019.11.26.
- Wolberg AS, Aleman MM, Leiderman K, Machlus KR. Procoagulant activity in hemostasis and thrombosis: Virchow's triad revisited. Analgesia 2012; 114(2):275-85. doi: 10.1213/ANE.0b013e31823a088c.
- Franchini M, Lippi G, Manzato F, Vescovi PP, Targher G. Hemostatic abnormalities in endocrine and metabolic disorders. Eur J Endocrinol 2010; 162(3):439-51. doi: 10.1530/EJE-09-0958.
- Allman-Farinelli MA. Obesity and venous thrombosis: A review. Semin Thromb Hemost 2011; 37(8):903-7. doi: 10.1055/s-0031-1297369.
- Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomised studies in meta-analyses. Eur J Epidemiol 2010; 25(9):603-5. doi: 10.1007/s10654-010-9491-z.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327(7414): 557-60. doi: 10.1136/bmj.327.7414.557.
- Vaya A, Martinez-Triguero ML, Espana F, Todoli JA, Bonet E, Corella D. The metabolic syndrome and its individual components: Its association with venous thromboembolism in a mediterranean population. Metab Syn Relat Disord 2011; 9(3):197-201. doi: 10.1089/met.2010.0117.
- Jang MJ, Choi WI, Bang SM, Lee T, Kim YK, Ageno W, et al. Metabolic syndrome is associated with venous thromboembolism in the Korean population. Arterioscler Thromb Vasc Biol 2009; 29(3):311-5. doi: 10.1161/ ATVBAHA.109.184085.
- Ay C, Tengler T, Vormittag R, Simanek R, Dorda W, Vukovich T, et al. Venous thromboembolism - a manifestation of the metabolic syndrome. Haematologica 2007; 92(3):374-380. doi: 10.3324/haematol.10828.
- Ambrosetti M, Ageno W, Salerno M, Pedretti RF, Salerno-Uriarte JA. Metabolic syndrome as a risk factor for deep vein thrombosis after acute cardiac conditions. Thromb Res 2007; 120(6):815-818. doi: 10.1016/j.thromres.2007. 02.005.
- Ageno W, Prandoni P, Romualdi E, Ghirarduzzi A, Dentali F, Pesavento R, et al. The metabolic syndrome and the risk of venous thrombosis: A case-control study. J Thromb Haemost 2006; 4(9):1914-8. doi: 10.1111/j.1538-7836. 2006.02132.x.
- Ray JG, Lonn E, Yi Q, Rathe A, Sheridan P, Kearon C, et al. Venous thromboembolism in association with features of the metabolic syndrome. QJM 2007; 100(11):679-84. doi: 10.1093/qjmed/hcm083.
- Steffen LM, Cushman M, Peacock JM, Heckbert SR, Jacobs DR, Rosamond WD, et al. Metabolic syndrome and risk of venous thromboembolism: Longitudinal investigation of thromboembolism etiology. J Thromb Haemost 2009; 7(5): 746-51. doi: 10.1111/j.1538-7836.2009.03295.x.
- Abdollahi M, Cushman M, Rosendaal FR. Obesity: Risk of venous thrombosis and the interaction with coagulation factor levels and oral contraceptive use. Thromb Haemost 2003; 89(3):493-8.
- Delluc A, Le Moigne E, Tromeur C, Noel-Savina E, Couturaud F, Mottier D, et al. Site of venous thromboembolism and prothrombotic mutations according to body mass index. Results from the EDITH study. Br J Haematol 2011; 154(4):486-91. doi: 10.1111/j.1365-2141. 2011.08592.x.
- Dentali F, Ageno W, Romualdi E, Pesavento R, Ghirarduzzi A, Prandoni P. Metabolic syndrome and hyper-homocysteinemia in patients with deep vein thrombosis: A case-control study. Haematologica 2007; 92(9):1293-1294. doi: 10.3324/haematol.11352.
- Di Minno MN, Tufano A, Rusolillo A, Di Minno G, Tarantino G. High prevalence of nonalcoholic fatty liver in patients with idiopathic venous thromboembolism. World J Gastroenterol 2010; 16(48):6119-22. doi: 10.3748/wjg. v16.i48.6119.
- Halvorson EE, Ervin SE, Russell TB, Skelton JA, Davis S, Spangler J. Association of obesity and pediatric venous thromboembolism. Hosp Pediatr 2016; 6(1):22-6. doi: 10.1542/hpeds.2015-0039.
- Huerta C, Johansson S, Wallander MA, Garcia Rodriguez LA. Risk factors and short-term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Arch Intern Med 2007; 167(9):935-43. doi: 10.1001/archinte.167.9.935.
- Lidegaard O, Edstrom B, Kreiner S. Oral contraceptives and venous thromboembolism: A five-year national case-control study. Contra 2002; 65(3):187-196. doi: 10.1016/s0010- 7824(01)00307-9.
- Nightingale AL, Lawrenson RA, Simpson EL, Williams TJ, MacRae KD, Farmer RD. The effects of age, body mass index, smoking and general health on the risk of venous thromboembolism in users of combined oral contra-ceptives. Eur J Contracept Reprod Health Care 2000; 5(4):265-74. doi: 10.1080/13625180008500402.
- Rosenfeld HE, Tsokos M, Byard RW. The association between body mass index and pulmonary thrombo-embolism in an autopsy population. J Fore Sci 2012; 57(5):1336-8. doi: 10.1111/j.1556-4029.2012.02140.x.
- Stokes S, Breheny P, Radulescu A, Radulescu VC. Impact of obesity on the risk of venous thromboembolism in an inpatient pediatric population. Pediatr Hematol Oncol 2014; 31(5):475-80. doi: 10.3109/08880018.2014.886315.
- Vayá A, Falcó C, Simó M, Ferrando F, Mira Y, Todolí J, et al. Influence of lipids and obesity on haemorheological parameters in patients with deep vein thrombosis. Thromb Haemost 2007; 98(3):621-6.
- Hong C, Zhu F, Du D, Pilgram TK, Sicard GA, Bae KT. Coronary artery calcification and risk factors for atherosclerosis in patients with venous thromboembolism. Atherosclerosis 2005; 183(1):169-74. doi: 10.1016/j. atherosclerosis.2005.03.047.
- Hotoleanu C. Association between obesity and venous thromboembolism. Med Pharm Rep 2020; 93(2):162-8. doi: 10.15386/mpr-1372.
- Fontaine GV, Vigil E, Wohlt PD, Lloyd JF, Evans RS, Collingridge DS, et al. Venous thromboembolism in critically Ill medical patients receiving chemoprophylaxis: A focus on obesity and other risk factors. Clin Appl Thromb Hemost 2016; 22(3):265-73. doi: 10.1177/1076029 615604048.
- Di Nisio M, Di Iorio A, Porreca E, Abate M, Ferrante N, Bandinelli S, et al. Obesity, poor muscle strength, and venous thromboembolism in older persons: The InCHIANTI study. J Gerontol A Biol Sci Med Sci 2011; 66(3):320-5. doi: 10.1093/gerona/glq207.
- Cushman M, O'Meara ES, Heckbert SR, Zakai NA, Rosamond W, Folsom AR. Body size measures, hemostatic and inflammatory markers and risk of venous thrombosis: The longitudinal investigation of thromboembolism etiology. Thromb Res 2016; 144:127-32. doi: 10.1016/j. thromres.2016.06.012.
- World Health Organization (2018) obesity and overweight fact Sheet. www.who.int/news-room/fact-sheets/detail/ obesity-and-overweight.
- Braekkan SK, Hald EM, Mathiesen EB, Njolstad I, Wilsgaard T, Rosendaal FR, et al. Competing risk of atherosclerotic risk factors for arterial and venous thrombosis in a general population: The tromso study. Arterioscler Thromb Vasc Biol 2012; 32(2):487-91. doi: 10.1161/ATVBAHA.111. 237545.
- Blokhin IO, Lentz SR. Mechanisms of thrombosis in obesity. Curr Opin Hematol 2013; 20(5):437-44. doi: 10.1097/MOH. 0b013e3283634443.
- Levi M, van der Poll T, Büller HR. Bidirectional relation between inflammation and coagulation. Circulation 2004; 109(22):2698-2704. doi:10.1161/01.CIR.0000131660. 51520.9A.
- Ziccardi P, Nappo F, Giugliano G, Esposito K, Marfella R, Cioffi M, et al. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circ 2002; 105(7):804-9. doi: 10.1161/hc0702.104279.
- Darvall KA, Sam RC, Silverman SH, Bradbury AW, Adam DJ. Obesity and thrombosis. Eur J Vasc Endovasc Surg 2007; 33(2):223-33. doi: 10.1016/j.ejvs.2006.10.006.
- Hunt BJ. Hemostasis at extremes of body weight. Semin Thromb Hemost 2018; 44(7):632-9. doi: 10.1055/s-0038- 1661385.
- Morange PE, Alessi MC. Thrombosis in central obesity and metabolic syndrome: Mechanisms and epidemiology. Thromb Haemost 2013; 110(4):669-80. doi: 10.1160/ TH13-01-0075.
- Van Guilder GP, Hoetzer GL, Greiner JJ, Stauffer BL, DeSouza CA. Metabolic syndrome and endothelial fibrinolytic capacity in obese adults. Am J Physiol Regul Integr Comp Physiol 2008; 294(1):R39-44. doi: 10.1152/ ajpregu.00564.2007.
- Alessi MC, Juhan-Vague I. PAI-1 and the metabolic syndrome: Links, causes, and consequences. Arterioscler Thromb Vasc Biol 2006; 26(10):2200-07. doi: 10.1161/01.ATV.0000242905.41404.68.
- Mertens I, Van Gaal LF. Obesity, haemostasis and the fibrinolytic system. Obes Rev 2002; 3(2):85-101. doi: 10.1046/j.1467-789x.2002.00056.x.
- Godsland IF, Crook D, Proudler AJ, Stevenson JC. Hemostatic risk factors and insulin sensitivity, regional body fat distribution, and the metabolic syndrome. J Clin Endocrinol Metab 2005; 90(1):190-7. doi: 10.1210/jc.2004- 1292.
- Dentali F, Squizzato A, Ageno W. The metabolic syndrome as a risk factor for venous and arterial thrombosis. Semin Thromb Hemost 2009; 35(5):451-7. doi: 10.1055/s-0029- 1234140.
- Lakoski SG, Savage PD, Berkman AM, Penalosa L, Crocker A, Ades PA, et al. The safety and efficacy of early-initiation exercise training after acute venous thromboembolism: A randomised clinical trial. J Thromb Haemost 2015; 13(7): 1238-44. doi: 10.1111/jth.12989.