- Department of Neurosurgery, Yale University School of Medicine, New Haven, Connecticut, United States.
- Department of Therapeutic Radiology, Yale University School of Medicine, New Haven, Connecticut, United States.
- Department of Pathology, Yale University School of Medicine, New Haven, Connecticut, United States.
Correspondence Address:
E. Zeynep Erson-Omay
Department of Neurosurgery, Yale University School of Medicine, New Haven, Connecticut, United States.
DOI:10.25259/SNI_265_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: . Hong Christopher S1, Adam J. Kundishora1, Aladine A. Elsamadicy1, Andrew B. Koo1, Jason M. Beckta2, Declan McGuone3, E. Zeynep Erson-Omay1, Sacit Bulent Omay1. Genetic characterization of a case of sellar metastasis from bronchial carcinoid neuroendocrine tumor. 25-Sep-2020;11:303
How to cite this URL: . Hong Christopher S1, Adam J. Kundishora1, Aladine A. Elsamadicy1, Andrew B. Koo1, Jason M. Beckta2, Declan McGuone3, E. Zeynep Erson-Omay1, Sacit Bulent Omay1. Genetic characterization of a case of sellar metastasis from bronchial carcinoid neuroendocrine tumor. 25-Sep-2020;11:303. Available from: https://surgicalneurologyint.com/surgicalint-articles/10290/
Abstract
Background: Metastasis to the pituitary gland from neuroendocrine tumors is a rare occurrence that may originate from primary tumors the lung, gastrointestinal tract, thyroid, and pancreas, among others. Patients may present with signs of endocrine dysfunction secondary to pituitary involvement, as well as mass effect-related symptoms including headaches and visual deficits. Despite a small but accumulating body of literature describing the clinical and histopathological correlates for pituitary metastases from neuroendocrine tumors, the genetic basis underlying this presentation remains poorly characterized.
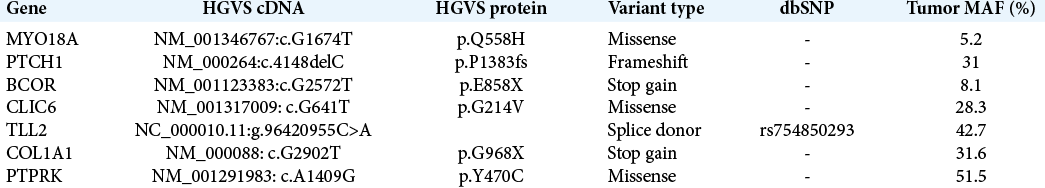
Case Description: We report the case of a 68-year-old with a history of lung carcinoid tumor who developed a suprasellar lesion, causing mild visual deficits but otherwise without clinical or biochemical endocrine abnormalities. She underwent endoscopic endonasal resection of her tumor with final pathology confirming metastasis from her original neuroendocrine tumor. Whole-exome sequencing was performed on the resected sellar tumor and matching blood, revealing increased genomic instability and key mutations in PTCH1 and BCOR that have been previously implicated in both systemic neuroendocrine and primary pituitary tumors with potentially actionable therapeutic targets.
Conclusion: This is the first genomic characterization of a metastatic tumor to the sella and reports potential genetic insight, implicating PTCH1 and BCOR mutations, into the pathophysiology of sellar metastasis from primary systemic tumors.
Keywords: Carcinoid, Metastasis, Neuroendocrine, Pituitary, Sella
INTRODUCTION
Metastases to the region of the sella remain uncommon, representing approximately 1% of all sellar tumors.[
While surgery and radiation remain front-line therapies for most sellar lesions, effective medical treatment, such as targeted therapies for hormonally active pituitary adenomas, is limited for other sellar pathologies. However, the recent success of small molecule inhibitors in BRAF-mutated craniopharyngioma has demonstrated the utility of comprehensive genetic analysis of challenging sellar pathologies.[
CASE DESCRIPTION
Clinical presentation
The patient is a 68-year-old female who was first diagnosed with a carcinoid tumor of the left lower lobe of the lung, diagnosed after lobectomy with 7/9 lymph nodes positive for tumor spread, consistent with pT2, N2 Grade 2 staging. She was treated with adjuvant etoposide and cisplatin, as well as concurrent fractionated radiation therapy (50 Gy in 25 fractions). Her clinical course remained uneventful, but due to rising surveillance chromogranin levels, she underwent a surveillance PET-CT 6 years later, which showed increased metabolic activity in the sellar region, despite being on octreotide therapy. This prompted brain magnetic resonance imaging, which demonstrated a 2.6 × 2.5 × 2 cm suprasellar mass, extending into the right cavernous sinus, with mass effect on the optic chiasm [
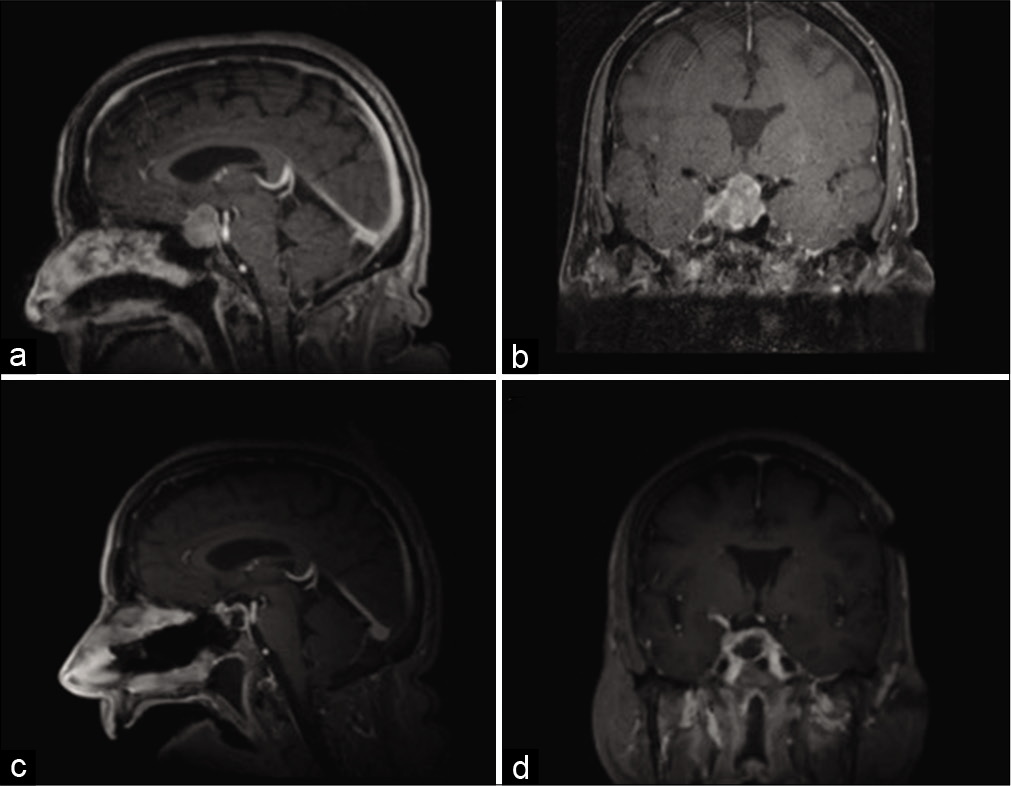
Figure 1:
Magnetic resonance imaging preoperatively and postoperatively. Representative (a) sagittal and (b) coronal images of T1-weighted postcontrast magnetic resonance imaging (MRI) are shown at time of presentation. Intraoperative MRI was obtained, demonstrative gross total resection of the sellar tumor, as seen on representative (c) sagittal and (d) coronal images of T-weighted postcontrast MRI.
Although a diagnosis of pituitary adenoma was suspected, given her cancer history, PET-CT findings, and rising chromogranin levels, metastasis remained in the differential diagnosis, and as such, she underwent endoscopic endonasal resection of her sellar tumor. Intraoperatively, the tumor was resected piecemeal and noted to have a separate plane from the normal appearing pituitary gland, which was kept intact. Intraoperative imaging showed gross total resection of the tumor [
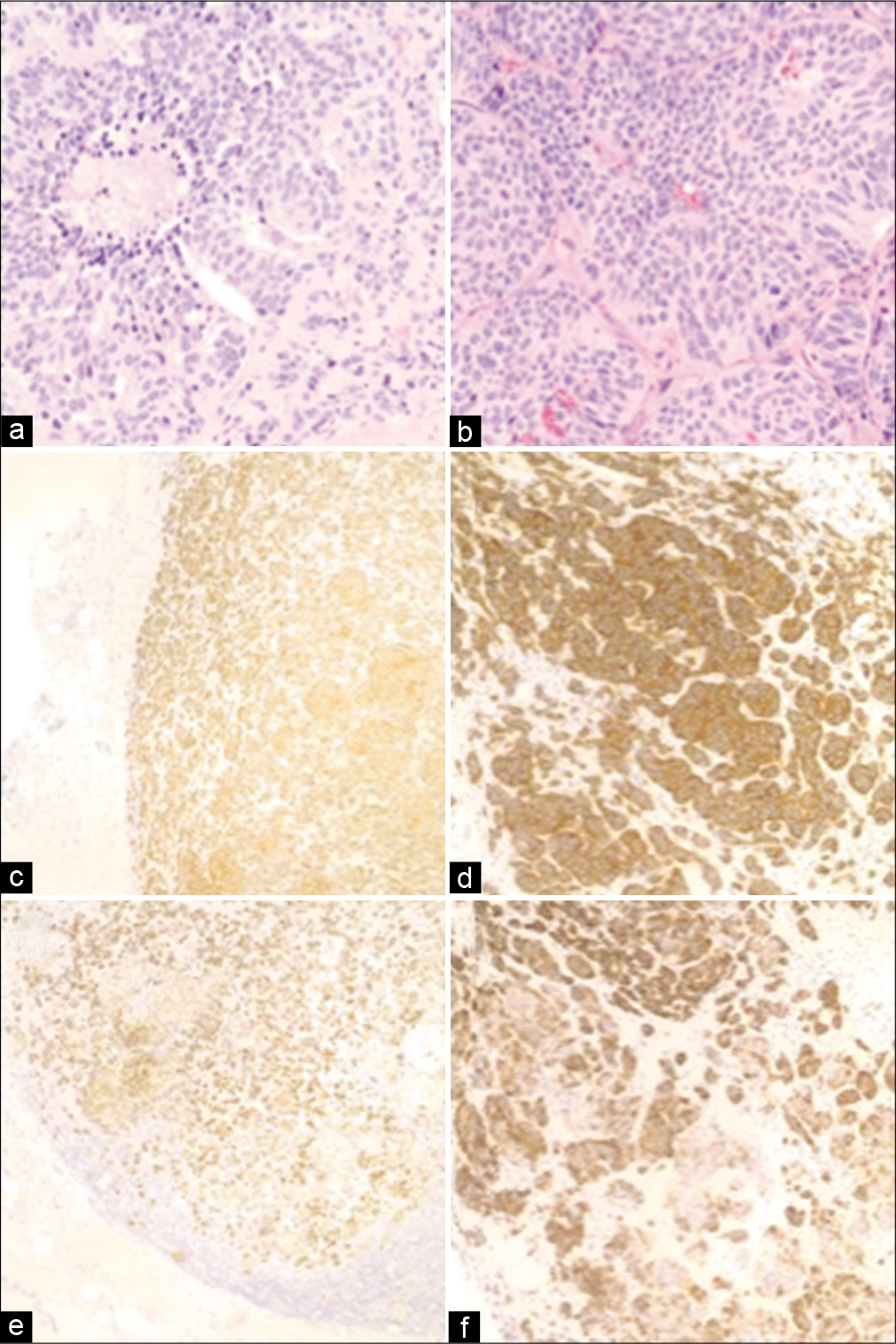
Figure 2:
Histopathology of primary lung and sellar metastasis specimens. Routine H&E stains from the (a) lung and (b) sellar tumors demonstrate elevated mitotic activity and areas of focal necrosis (×400). Neuroendocrine origin was confirmed through positive immunohistochemistry for synaptophysin in the (c) lung (×100) and (d) sellar tumors (×200), as well as for CK7 in the (e) lung (×100) and (f) sellar tumors (×200).
Given the high proliferative index of the tumor, the patient underwent adjuvant stereotactic radiosurgery 2 months after resection. Late postoperative endocrine evaluation demonstrated central hypothyroidism, for which she was started on levothyroxine therapy. She continues to be monitored for signs of hypoadrenalism.
Genomic analysis
WES was performed on the resected sellar tumor and the matching blood sample [
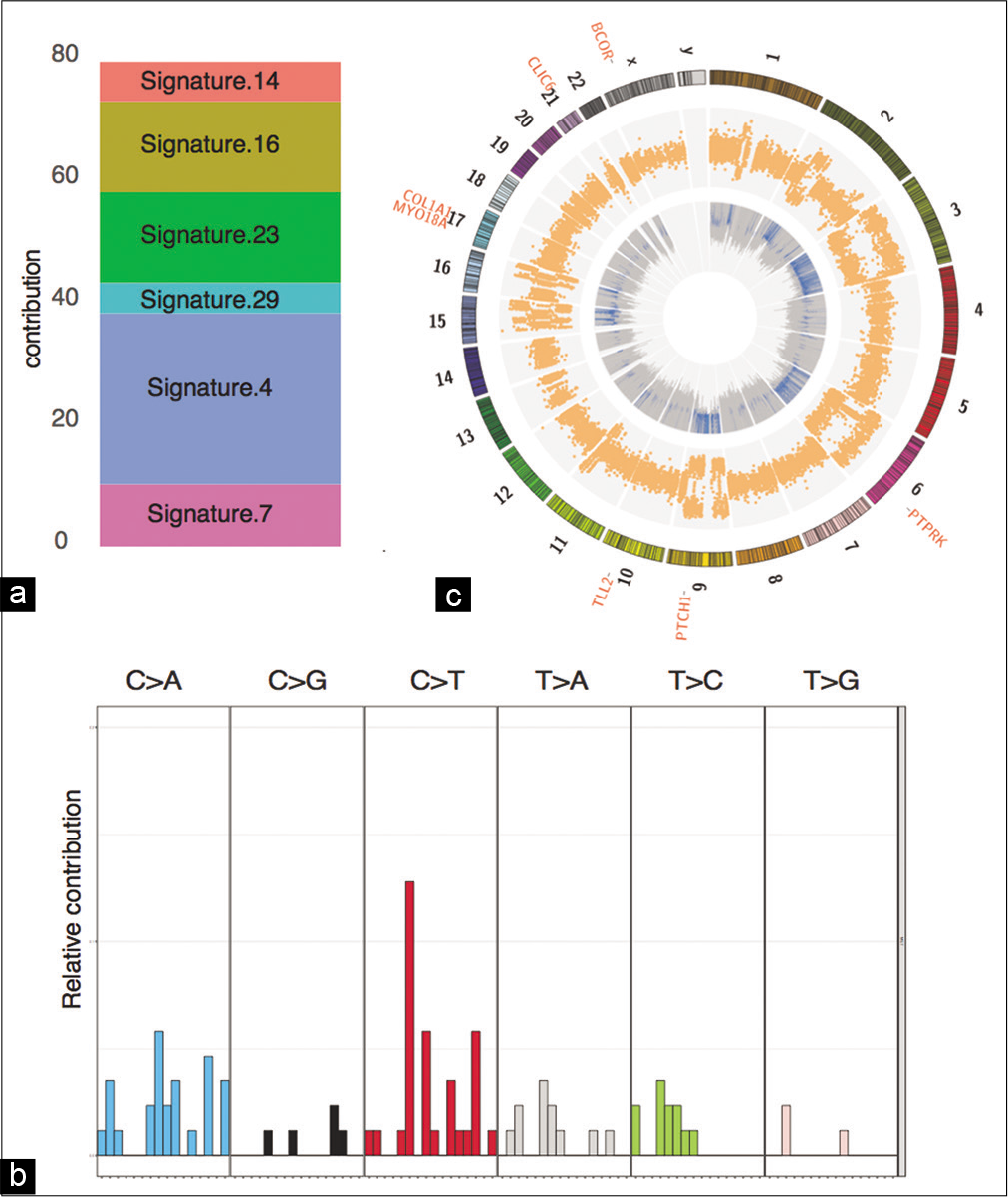
Figure 3:
Genomics summary of the sellar specimen. (a) Mutation signature contribution to COSMIC signatures. (b) Distribution of 96 mutation signatures. (c) Circos plot depicting the CNV and LOH events in the inner and outer circles, respectively. The cancer-associated somatic mutations are listed outside the karyotype.
DISCUSSION
As patients continue to live longer with advances in systemic therapy, metastases to the sellar region, while still rare, must remain in the differential of new sellar lesions in patients with a known oncologic history. The most common origins of sellar metastases in women originate from the breast and lung in men.[
The genetic changes underlying sellar metastases from systemic cancer remain poorly characterized with no prior reports in the literature to our knowledge. We performed WES of the sellar metastasis in our patient, which revealed various genetic alterations, supporting initial neuroendocrine origin. We found a deleterious mutation of PTCH1, which encodes patched-1 protein, a receptor for sonic hedgehog (SHH) signaling that suppresses the release of SMO (smoothened)-mediated cell proliferation, thus acting as a tumor suppressor.[
Interestingly, some of the mutations detected in the sellar metastasis have been previously implicated in normal pituitary development and pathology. PTCH1 has been shown to be important in pituitary embryogenesis, giving rise to normal pituitary development through proliferation of normal Rathke’s pouch progenitors and differentiation of pituitary cell types.[
While the genetic findings of this study are unique, the clinical implications remain unclear, as there is a paucity of data in the literature regarding systemic therapies that target specific mutations in sellar tumors and are limited to preliminary clinical studies, most notably the use of BRAF inhibitors in BRAF-mutated craniopharyngiomas.[
This study was limited by the ability to perform comparable genomic analysis of the original pulmonary carcinoid specimen, which was not possible due to lack of available tissue. As such, we were not able to map the genetic evolution of the tumor from initial origin to distant recurrence in the sellar region. However, WES from the resected tumor did provide novel genetic insights into the pathophysiology of sellar metastasis from systemic tumors and may guide further avenues for research.
CONCLUSION
Sellar metastases from primary systemic tumors represent a small but growing pathology that arise as patients continue to live longer with modern oncologic therapies. While clinical features exist that may help differentiate patients with metastases from primary pituitary adenomas at time of presentation, such as the presence of diabetes insipidus, surgical intervention remains critical for definitive diagnosis to guide further therapy and to relieve symptoms secondary to endocrine dysfunction, or mass effect on nearby cranial nerves. Genetic alterations underlying metastasis to the sellar region remain uncharacterized, but this report highlights a potential key role for mutations in PTCH1 and BCOR, previously implicated in both primary neuroendocrine and pituitary tumors. Further genetic studies of these rare cases are needed to elucidate the mechanisms underlying sellar metastasis of systemic cancers.
Ethics approval
Genomic analysis was performed after consenting the patient to an institutional review board-approved protocol for whole-exome sequencing of resected brain tumor specimens.
Availability of data and material
The reported variants were submitted to ClinVar (
Declaration of patient consent
Institutional Review Board permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Adolphe C, Hetherington R, Ellis T, Wainwright B. Patched1 functions as a gatekeeper by promoting cell cycle progression. Cancer Res. 2006. 66: 2081-8
2. Agarwal S, Al-Keilani MS, Alqudah MA, Sibenaller ZA, Ryken TC, Assem M. Tumor derived mutations of protein tyrosine phosphatase receptor type k affect its function and alter sensitivity to chemotherapeutics in glioma. PLoS One. 2013. 8: e62852
3. Astolfi A, Fiore M, Melchionda F, Indio V, Bertuccio SN, Pession A. BCOR involvement in cancer. Epigenomics. 2019. 11: 835-55
4. Aydin B, Arga KY. Co-expression network analysis elucidated a core module in association with prognosis of non-functioning non-invasive human pituitary adenoma. Front Endocrinol (Lausanne). 2019. 10: 361
5. Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015. 5: 1164-77
6. Brastianos PK, Shankar GM, Gill CM, Taylor-Weiner A, Nayyar N, Panka DJ. Dramatic response of BRAF V600E mutant papillary craniopharyngioma to targeted therapy. J Natl Cancer Inst. 2016. 108: djv310
7. Carreno G, Apps JR, Lodge EJ, Panousopoulos L, Haston S, Gonzalez-Meljem JM. Hypothalamic sonic hedgehog is required for cell specification and proliferation of LHX3/LHX4 pituitary embryonic precursors. Development. 2017. 144: 3289-302
8. Chiang MF, Brock M, Patt S. Pituitary metastases. Neurochirurgia (Stuttg). 1990. 33: 127-31
9. Dummer R, Ascierto PA, Basset-Seguin N, Dreno B, Garbe C, Gutzmer R. Sonidegib and vismodegib in the treatment of patients with locally advanced basal cell carcinoma: A joint expert opinion. J Eur Acad Dermatol Venereol. 2020. 34: 1944-56
10. Forbes SA, Beare D, Gunasekaran P, Leung K, Bindal N, Boutselakis H. COSMIC: Exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015. 43: D805-11
11. George J, Lim JS, Jang SJ, Cun Y, Ozretic L, Kong G. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015. 524: 47-53
12. Goglia U, Ferone D, Sidoti M, Spaziante R, Dadati P, Ravetti JL. Treatment of a pituitary metastasis from a neuroendocrine tumour: Case report and literature review. Pituitary. 2008. 11: 93-102
13. Gomes DC, Jamra SA, Leal LF, Colli LM, Campanini ML, Oliveira RS. Sonic hedgehog pathway is upregulated in adamantinomatous craniopharyngiomas. Eur J Endocrinol. 2015. 172: 603-8
14. Gurung B, Hua X, Runske M, Bennett B, LiVolsi V, Roses R. PTCH 1 staining of pancreatic neuroendocrine tumor (PNET) samples from patients with and without multiple endocrine neoplasia (MEN-1) syndrome reveals a potential therapeutic target. Cancer Biol Ther. 2015. 16: 219-24
15. He W, Chen F, Dalm B, Kirby PA, Greenlee JD. Metastatic involvement of the pituitary gland: A systematic review with pooled individual patient data analysis. Pituitary. 2015. 18: 159-68
16. Juratli TA, Jones PS, Wang N, Subramanian M, Aylwin SJB, Odia Y. Targeted treatment of papillary craniopharyngiomas harboring BRAF V600E mutations. Cancer. 2019. 125: 2910-4
17. Katoh M. Genomic testing, tumor microenvironment and targeted therapy of Hedgehog-related human cancers. Clin Sci (Lond). 2019. 133: 953-70
18. Komninos J, Vlassopoulou V, Protopapa D, Korfias S, Kontogeorgos G, Sakas DE. Tumors metastatic to the pituitary gland: Case report and literature review. J Clin Endocrinol Metab. 2004. 89: 574-80
19. Li F, Sun L, Zhang S. Acquirement of DNA copy number variations in non-small cell lung cancer metastasis to the brain. Oncol Rep. 2015. 34: 1701-7
20. Max MB, Deck MD, Rottenberg DA. Pituitary metastasis: Incidence in cancer patients and clinical differentiation from pituitary adenoma. Neurology. 1981. 31: 998-1002
21. McArdle L, Rafferty M, Maelandsmo GM, Bergin O, Farr CJ, Dervan PA. Protein tyrosine phosphatase genes downregulated in melanoma. J Invest Dermatol. 2001. 117: 1255-60
22. Moshkin O, Rotondo F, Scheithauer BW, Soares M, Coire C, Smyth HS. Bronchial carcinoid tumors metastatic to the sella turcica and review of the literature. Pituitary. 2012. 15: 160-5
23. Nakamura M, Kishi M, Sakaki T, Hashimoto H, Nakase H, Shimada K. Novel tumor suppressor loci on 6q22-23 in primary central nervous system lymphomas. Cancer Res. 2003. 63: 737-41
24. Paret C, Theruvath J, Russo A, Kron B, El Malki K, Lehmann N. Activation of the basal cell carcinoma pathway in a patient with CNS HGNET-BCOR diagnosis: Consequences for personalized targeted therapy. Oncotarget. 2016. 7: 83378-91
25. Park C, Ha SY, Kim ST, Kim HC, Heo JS, Park YS. Identification of the BRAF V600E mutation in gastroenteropancreatic neuroendocrine tumors. Oncotarget. 2016. 7: 4024-35
26. Peifer M, Fernandez-Cuesta L, Sos ML, George J, Seidel D, Kasper LH. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet. 2012. 44: 1104-10
27. Rudin CM, Durinck S, Stawiski EW, Poirier JT, Modrusan Z, Shames DS. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet. 2012. 44: 1111-6
28. Schubiger O, Haller D. Metastases to the pituitary-hypothalamic axis, An MR study of 7 symptomatic patients. Neuroradiology. 1992. 34: 131-4
29. Shida T, Furuya M, Nikaido T, Hasegawa M, Koda K, Oda K. Sonic hedgehog-gli1 signaling pathway might become an effective therapeutic target in gastrointestinal neuroendocrine carcinomas. Cancer Biol Ther. 2006. 5: 1530-8
30. Siqueira PF, Mathez AL, Pedretti DB, Abucham J. Pituitary metastasis of lung neuroendocrine carcinoma: Case report and literature review. Arch Endocrinol Metab. 2015. 59: 548-53
31. Starr TK, Allaei R, Silverstein KA, Staggs RA, Sarver AL, Bergemann TL. A transposon-based genetic screen in mice identifies genes altered in colorectal cancer. Science. 2009. 323: 1747-50
32. Sun PH, Ye L, Mason MD, Jiang WG. Protein tyrosine phosphatase kappa (PTPRK) is a negative regulator of adhesion and invasion of breast cancer cells, and associates with poor prognosis of breast cancer. J Cancer Res Clin Oncol. 2013. 139: 1129-39
33. Thompson EM, Ashley D, Landi D. Current medulloblastoma subgroup specific clinical trials. Transl Pediatr. 2020. 9: 157-62
34. Vila G, Theodoropoulou M, Stalla J, Tonn JC, Losa M, Renner U. Expression and function of sonic hedgehog pathway components in pituitary adenomas: Evidence for a direct role in hormone secretion and cell proliferation. J Clin Endocrinol Metab. 2005. 90: 6687-94
35. Wu Y, Yue L, Li J, Yuan M, Chai Y. Cushing’s syndrome secondary to typical pulmonary carcinoid with mutation in BCOR gene: A case report. Medicine (Baltimore). 2017. 96: e7870
36. Yin M, Chung YJ, Lindsley RC, Walker RL, Zhu YJ, Ebert BL. Engineered Bcor mutations lead to acute leukemia of progenitor B-1 lymphocyte origin in a sensitized background. Blood. 2019. 133: 2610-4