- Department of Neurosurgery, Atsuchi Neurosurgical Hospital,
- Department of Neurosurgery, Kagoshima University, Kagoshima City, Japan,
- Department of Neurologic Surgery, Mayo Clinic, Rochester, Minnesota, United States,
- Department of Neurosurgery, Kyushu University, Higashi-ku, Hukuoka, Japan.
Correspondence Address:
Kazunori Arita, Department of Neurosurgery, Kagoshima University, Kagoshima City, Japan.
DOI:10.25259/SNI_291_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Takashi Kawahara1, Masamichi Atsuchi1, Kazunori Arita2, Shingo Fujio2, Nayuta Higa2, FM Moinuddin3, Koji Yoshimoto4, Ryosuke Hanaya2. Dural sac shrinkage signs on spinal magnetic resonance imaging indicate overdrainage after lumboperitoneal shunt for idiopathic normal pressure hydrocephalus. 23-Jun-2022;13:269
How to cite this URL: Takashi Kawahara1, Masamichi Atsuchi1, Kazunori Arita2, Shingo Fujio2, Nayuta Higa2, FM Moinuddin3, Koji Yoshimoto4, Ryosuke Hanaya2. Dural sac shrinkage signs on spinal magnetic resonance imaging indicate overdrainage after lumboperitoneal shunt for idiopathic normal pressure hydrocephalus. 23-Jun-2022;13:269. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11676
Abstract
Background: We previously found the usefulness of dural sac shrinkage signs (DSSSs), which are the anterior shift of the spinal cord and dura mater behind the cord, detected by magnetic resonance imaging (MRI) at the thoracic level for the diagnosis of spontaneous intracranial hypotension (IH). This is a retrospective survey on the usefulness of DSSSs for the early detection of iatrogenic IH caused by overdrainage through a lumboperitoneal shunt (LPS) for patients with idiopathic normal pressure hydrocephalus (INPH).
Methods: Forty-five INPH patients had an LPS using a pressure programmable valve equipped with an anti-siphon device.
Results: Nine patients complained of orthostatic headache after the LPS, indicating IH due to overdrainage, which persisted for more than a week in three patients and 2–7days in six patients. The headache was transient/ nonorthostatic in ten patients and absent in 26 patients. The DSSSs and accompanying enlargement of the venous plexus were observed in all three patients with prolonged orthostatic headaches. Only the anterior shift of the dura mater was observed in 1 (4%) among 25 patients who had short-term orthostatic headache, transient/ nonorthostatic headache, or absent headache, and underwent spinal MRI. A patient with prolonged severe orthostatic headache with both DSSSs eventually developed intracranial subdural effusion and underwent tandem valve surgery, which provided a quick improvement of symptoms. The DSSSs on thoracic MRI also disappeared promptly.
Conclusion: DSSSs may serve as objective signs for the diagnosis of IH due to overdrainage through an LPS for INPH.
Keywords: Dural sac shrinkage signs, Enlarged venous plexus, Lumboperitoneal shunt, Normal pressure hydrocephalus, Overdrainage
INTRODUCTION
Lumboperitoneal shunt (LPS) is an effective treatment for idiopathic normal pressure hydrocephalus (INPH). Intracranial hypotension (IH) caused by overdrainage through LPS presents with orthostatic headache, nuchal pain, vertigo, and nausea, which delay ambulation and prolongs hospital stay. A Japanese multicenter study found that approximately 30% of patients complained of symptoms of overdrainage after surgery.[
To prevent overdrainage, the authors routinely use a pressure programmable valve initially set at the highest opening pressure and equipped with an anti-siphon device.[
The authors previously reported highly sensitive and specific signs of spontaneous IH called dural sac shrinkage signs (DSSSs), which are the anterior shift of the spinal cord and dura mater behind the cord and are often accompanied by a prominent epidural venous plexus.[
We supposed the DSSSs may also provide good clues to diagnose secondary IH. Thus, we recently assessed the thoracic MRI scans of patients with post-LPS orthostatic headaches for DSSSs for the early detection of secondary IH due to overdrainage. This is a retrospective analysis of thoracic MRI scans of patients who underwent LPS in a single center for INPH.
MATERIALS AND METHODS
Patients
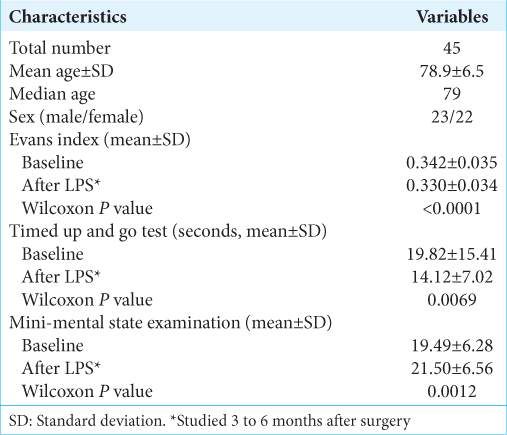
We retrospectively reviewed the charts and the neuroimaging database of 45 consecutive INPH patients who had an LPS during 10 months from July 2020 to April 2021 at the NPH Center in Atsuchi Neurosurgical Hospital, Kagoshima, Japan. The patient demographics are shown in
Neuroradiological studies
Brain MRI was obtained before the tap test using 3-T or 1.5-T MR scanners in all patients. The basic imaging sequences for brain MRI were axial T1-weighted imaging, T2-weighted imaging, fluid-attenuated inversion recovery, and diffusion-weighted imaging. T2 and T2-fat saturated spinal images were obtained in all to screen the spinal lesions which may be a possible cause of present difficulty in ambulation and could be aggravated by LPS.[
Definition of DSSS
The DSSSs were judged positive when the spinal cord and dura mater behind the spinal cord shifted anteriorly at the thoracic level. Based on the previous report, the anterior shift of the spinal cord was considered positive when the distance from the vertebral body to the anterior aspect of the spinal cord was <2 mm.[
Surgical procedures
All 45 patients had an LPS under neuroleptanalgesia and local anesthesia with a minimally invasive technique as previously reported.[
Clinical evaluation of postoperative overdrainage
In this study, IH symptoms due to overdrainage after the LPS were surrogated by the most frequent and specific one, which was orthostatic headache. The degree of headache was determined as follows: absent: no headache at all; transient/ nonorthostatic: transient and nonorthostatic headache that subsided within 2 days without special treatment; short orthostatic: orthostatic headache that improved within a week with bed resting and hydration; and prolonged orthostatic: severe orthostatic headache persisting for more than a week even with strict bed resting and sufficient hydration.
Statistical analysis
All the statistical analyses were conducted using StatFlex version 6.0 software (Artech Co., Ltd., Osaka, Japan). Depending on the characteristics of the data sets, the data were analyzed with Fisher’s exact test or the Wilcoxon signed-rank test. Statistical significance was set at P < 0.05.
Ethical consideration
This noninterventional study was approved by the Medical Ethics Committee of in Atsuchi Neurosurgical Hospital (R3-2, October 2021). This study was conducted according to the principles of the Declaration of Helsinki, as revised in 2000, and the Ethical Guidelines for Medical and Health Research Involving Human Subjects (effective on February 9, 2015) promulgated by the Ministry of Health, Labour and Welfare of Japan.
RESULTS
Efficacy of LPS
The mean baseline Evans index was 0.342 ± 0.035 (SD); it significantly decreased to 0.330 ± 0.034 (SD) 3–6 months after the LPS surgery (Wilcoxon’s P < 0.0001) [
Degree of headache after LPS
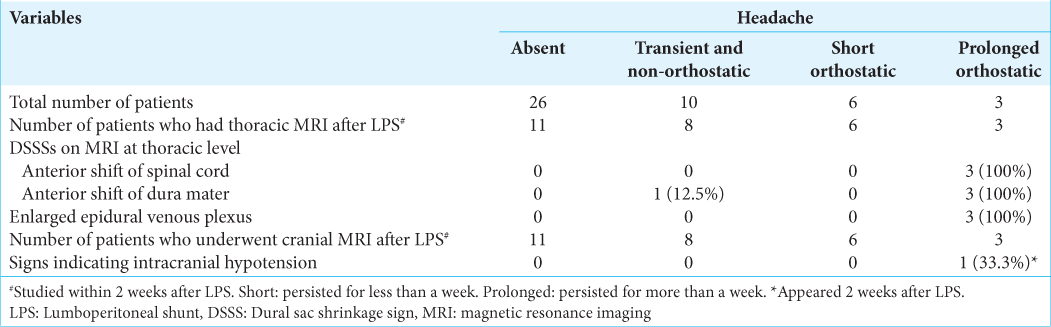
The number of patients with each category of headache was as follows; three had prolonged orthostatic, six had short orthostatic, ten had transient/nonorthostatic, and the remaining 26 patients had no headache [
Neuroradiological findings
Before LPS, spinal MRI showed only the anterior shift of the dura mater in 4 (8.9%) among 45 total patients. The incidence was 4% (1/25) before tap test and 15% (3/20) after tap test.
Spinal and brain MRI was obtained 2–14 days after the LPS for 28 patients: all three patients with prolonged orthostatic headache, all six patients with short orthostatic headache, eight of the ten patients with transient/nonorthostatic headache, and 11 of the 26 patients without headache [
Post-LPS brain MRI for the 29 patients showed no classic signs of IH except the representative case.
Representative case
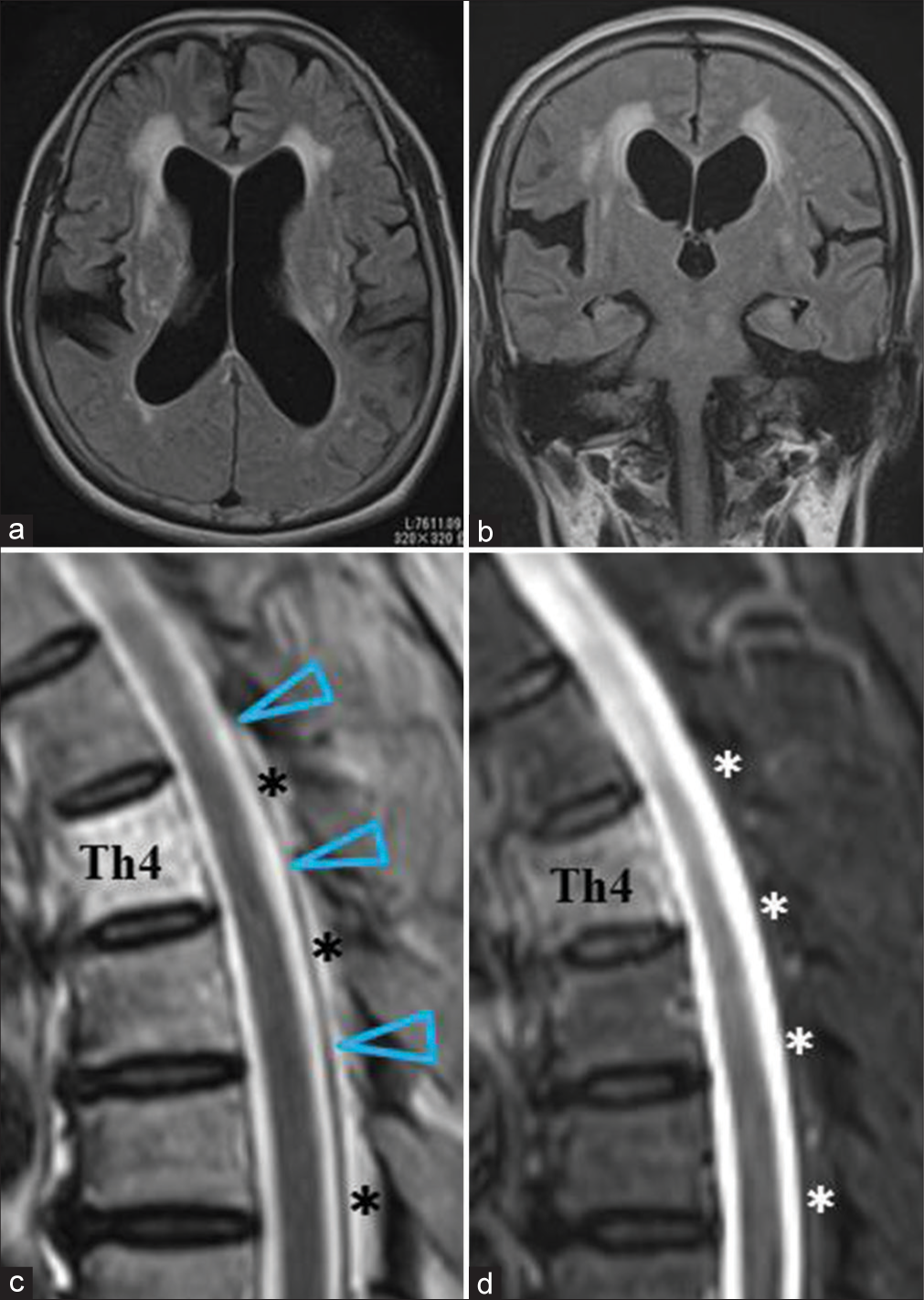
A 71-year-old female visited our clinic with complaints of gradually aggravating gait disturbance, memory disturbance, and urinary incontinence for the past 3 years. The MMSE score was 25/30. She was diagnosed with probable INPH based on the symptom triad, disproportionately enlarged subarachnoid space hydrocephalus on MRI [
Figure 1:
Preoperative magnetic resonance imaging (MRI) for a 71-year-old female with idiopathic normal pressure hydrocephalus. (a) Brain axial fluid-attenuated inversion recovery (FLAIR) image. (b) Brain coronal FLAIR image. (c) T2-weighted (T2-W) sagittal image at the thoracic level. (d) T2 fat-saturated sagittal image at the thoracic level. Brain MRIs showed enlarged lateral ventricles, unilateral dilatation of the right Sylvian fissure, and tight high-convexity sulci (a and b). T2-W sagittal imaging at the thoracic level showed normal positioning of the spinal cord and dura mater (arrowheads in [c]). Asterisks indicate epidural fat tissue (c and d). The Th4 vertebral body was involved by a hemangioma in this case.
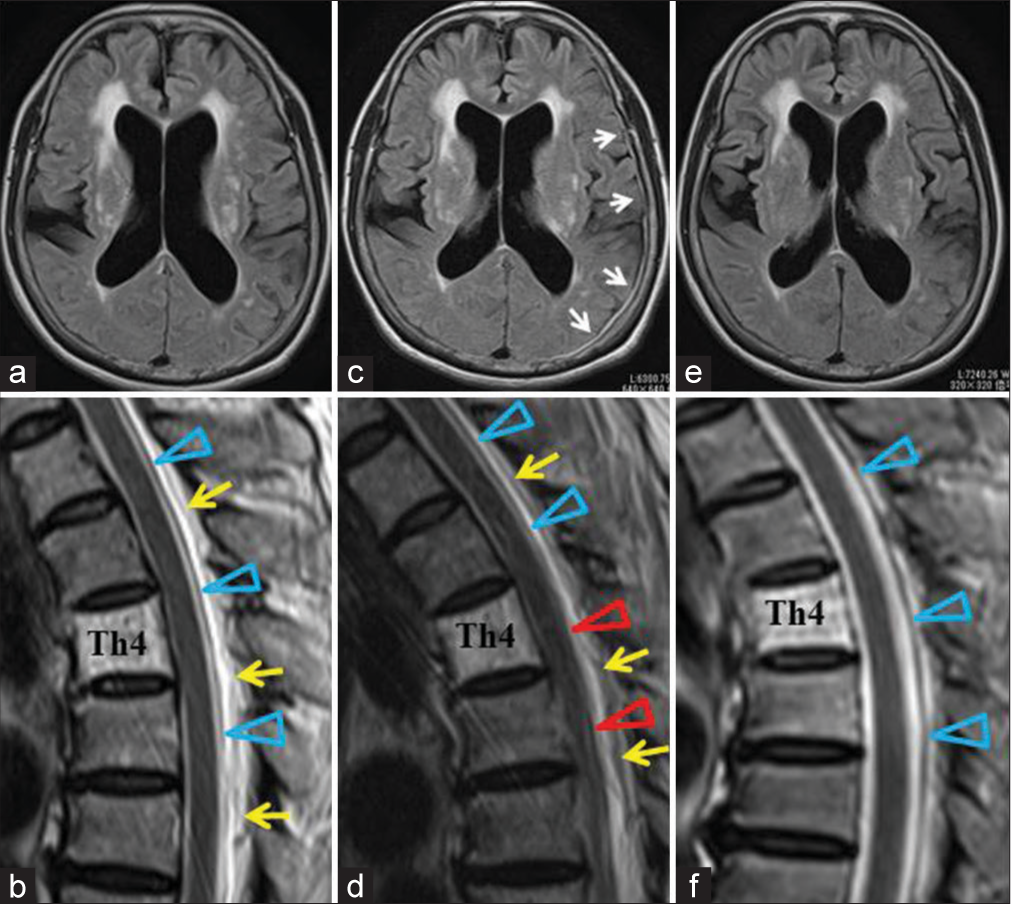
Postoperatively, she complained of severe orthostatic headache, nausea, and nuchal pain. Although brain MRI obtained 3 days after the surgery did not show any abnormality except the slight reduction of the ventricular size [
Figure 2:
MRIs after lumboperitoneal shunt (LPS) in a 71-year-old female complaining of a severe orthostatic headache after surgery. Upper column (a, c, e): Brain axial fluid-attenuated inversion recovery images. Lower column (b, d, f): T2-W sagittal images at the thoracic level. 3 days after LPS, brain MRI (a) showed no signs of intracranial hypotension IH. Thoracic MRI (b) revealed DSSSs, which are the anterior shift of spinal cord and dura mater (arrowheads), and enlarged epidural venous plexus (arrows). Two weeks after LPS, thoracic MRI (d) showed aggravation of the DSSSs. The spinal cord appeared to touch the vertebral body, and the dura mater (arrowheads) became inseparable from the spinal cord at the Th4-Th7 levels (red arrowheads). The epidural venous plexus was now engorged (arrows). Brain MRI (c) revealed a thin subdural effusion in the left convexity (white arrows). Six days after the tandem valve surgery, brain MRI showed the disappearance of subdural effusion (e). Spinal MRI (f) showed a normally positioned spinal cord and dura mater (arrowheads).
She was judged to suffer severe overdrainage. On the 16th day after the LPS, tandem valve surgery involving the vertical insertion of an additional valve (SHUNTASSISTANT, Aesculap, PA, USA) downstream to the previously installed valve was performed.[
After the surgery, the postural headache quickly disappeared. Brain and spinal MRIs 6 days after the tandem valve surgery revealed the disappearance of the overdrainage signs; subdural effusion, DSSSs, and dilatation of the epidural venous plexus [
DISCUSSION
The authors previously reported that the DSSSs on thoracic MRI are sensitive and specific markers of spontaneous IH.[
One-third of the patients who received LPS for the INPH were reported to suffer from the symptoms related to IH due to overdrainage including orthostatic headache.[
Several measures were proposed to prevent this complication, such as the use of a pressure controllable valve, anti-siphon device, tandem valve, and sufficient intravenous hydration for the patients after surgery. We currently use the pressure programmable valve equipped with an anti-siphon device initially set at the highest opening pressure of 40 cm H2O or more.[
Brain MRI during the early stage usually fails to show the classic signs of IH, such as downward displacement of the brain, subdural fluid collection, and pituitary enlargement. We found no classic signs of IH on brain MRI in nine patients with orthostatic headache, while thoracic MRI showed DSSSs and a dilated epidural venous plexus in all three patients with prolonged headaches out of these nine patients.
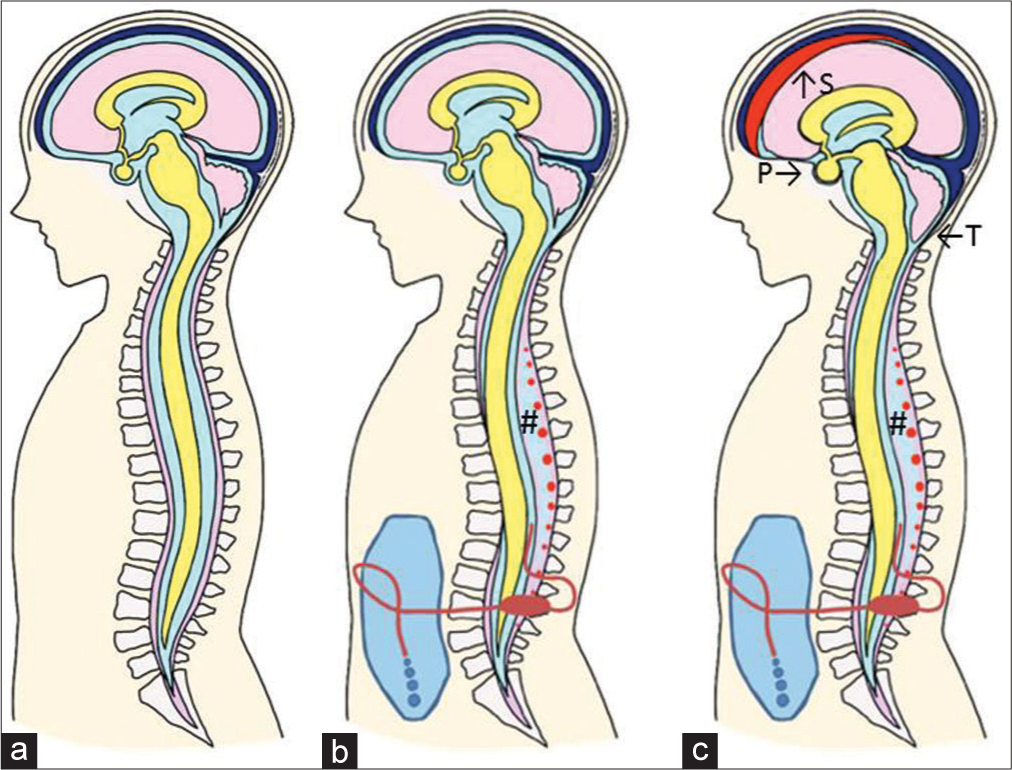
Our proposed mechanism underlying DSSSs in patients who had an LPS is as follows [
Figure 3:
Illustrative mechanism underlying the DSSSs and classic intracranial signs of IH after LPS. (a) Preoperative condition. (b) For the cases with overdrainage, the DSSSs, which are the anterior shift of the spinal cord and dura mater due to severe reduction of cerebrospinal fluid in the dural sac, develop. And the epidural venous plexus (red dots) is enlarged in low-pressure epidural space (#). Intracranial signs of IH are not seen at this stage. (c) When the overdrainage is prolonged, the classic intracranial signs of IH appear. S: Subdural effusion, P: Enlargement of the pituitary gland, and T: Herniation of cerebellar tonsil.
The collapse of the dural sac is compensated for by the enlargement of the surrounding venous plexus, as explained by the extended idea of the Monro-Kellie doctrine to the spinal column.[
Finding the DSSSs and enlargement of the epidural venous plexus after LPS are particularly important in patients who have difficulty in speech communication due to aphasia or severe cognitive decline. The absence of complaints of orthostatic headache in these patients underscores the value of DSSSs as objective indicators for the early detection of overdrainage.
The enlargement of the epidural venous plexus has been reported to occur in patients with overdrainage after the ventriculoperitoneal (VP) shunt, not the LP shunt. In these reports, an enlarged epidural venous plexus mainly observed at the cervical level with compression of the spinal cord caused myelopathy and occasional radiculopathy. It was designated as overshunting-associated myelopathy (OSAM) syndrome.[
Limitation of this study
This is the first study to investigate thoracic MRI in patients who had LPS for INPH, which showed that DSSSs were good indicators of overdrainage. However, the study had several limitations. First, 44.4% of the preoperative thoracic MRI scans were performed after tap rest. The transient in IH caused by the tapping could be a factor for the relatively high frequency (15%) of the anterior shift of dura mater in this group. Second, due to the retrospective nature of the study, we were able to analyze the postoperative spinal MRI of 62.2% (28/45) of the total participants. However, the DSSSs were observed only in 1 patient (4%) from the 25 patients with short orthostatic, transient/nonorthostatic, or absent headache. Compared with these groups, the DSSSs in patients with prolonged orthostatic headache were significantly frequent, 100% (3/3). The difference should be confirmed with a prospective study necessitating routine pretapping and postoperative thoracic MRIs for all study participants.
Third, only three patients complained of prolonged orthostatic headache in this series. The use of the pressure programmable valve set at the highest opening pressure, 40 cm H2O or more, which is equipped with an anti-siphon device in our center led to the scarcity of severe overdrainage symptoms. A multi-institutional study involving a larger sample of patients with prolonged overdrainage symptoms is needed to validate our discovery.
Fourth, we assumed entangled fascicular objects behind the dura mater, low intensity on T2-W thoracic MRI, and to be flow-void of enlarged epidural venous plexus in this and the previous study.[
Fifth, investigating the possible cause of the difference in the emergence of IH after the same procedure for LPS seems beyond the aim of this study. However, finding the factor causing the difference may confer the better management of patients after LPS surgery. At present, we are investigating the body habitus and size of spinal canal and intervertebral foramen in patients who underwent the LPS. We hope that we can reveal the possible anatomical background which leads to IH in the near future.
CONCLUSION
Based on a previous report that DSSSs and the enlargement of the epidural venous plexus at the thoracic level were sensitive and specific signs of IH, we performed this retrospective survey on the significance of these signs among 45 patients who received an LPS in our NPH center. We found these signs as good indicators for the early detection of overdrainage or iatrogenic IH after receiving an LPS. A multicenter prospective study is required to validate our findings. A comparison of the signs associated with LP and VP shunts is also warranted.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank Ms. Rina Kawahara for the comprehensive illustration [Figure 3], Haibunsha Kagoshima for performing a critical review of this manuscript, and Editage (www.editage.com ) for English language editing.
References
1. Aihara Y, Kawamata T, Mitsuyama T, Hori T, Okada Y. Novel method for controlling cerebrospinal fluid flow and intracranial pressure by use of a tandem shunt valve system. Pediatr Neurosurg. 2010. 46: 12-8
2. Aschoff A, Kremer P, Benesch C, Fruh K, Klank A, Kunze S. Overdrainage and shunt technology. A critical comparison of programmable, hydrostatic and variable-resistance valves and flow-reducing devices. Childs Nerv Syst. 1995. 11: 193-202
3. Caruso R, Wierzbicki V, Marrocco L, Pesce A, Piccione E. A poorly known cerebrospinal fluid shunt complication: Miyazaki syndrome. World Neurosurg. 2015. 84: 834-8
4. Dobrocky T, Grunder L, Breiding PS, Branca M, Limacher A, Mosimann PJ. Assessing spinal cerebrospinal fluid leaks in spontaneous intracranial hypotension with a scoring system based on brain magnetic resonance imaging findings. JAMA Neurol. 2019. 76: 580-7
5. Haacke EM, Xu Y, Cheng YC, Reichenbach JR. Susceptibility weighted imaging (SWI). Magn Reson Med. 2004. 52: 612-8
6. Hashimoto M, Ishikawa M, Mori E, Kuwana N. Diagnosis of idiopathic normal pressure hydrocephalus is supported by MRI-based scheme: A prospective cohort study. Cerebrospinal Fluid Res. 2010. 7: 18
7. 8. Ishizaka K, Kudo K, Fujima N, Zaitsu Y, Yazu R, Tha KK. Detection of normal spinal veins by using susceptibility-weighted imaging. J Magn Reson Imaging. 2010. 31: 32-8 9. Kawahara T, Arita K, Fujio S, Hanaya R, Atsuchi M, Moinuddin FM. Dural sac shrinkage signs on magnetic resonance imaging at the thoracic level in spontaneous intracranial hypotension its clinical significance. Acta Neurochir. 2021. 163: 2685-94 10. Kawahara T, Tokimura H, Higa N, Hirano H, Bohara M, Hanaya R. Surgical technique for preventing subcutaneous migration of distal lumboperitoneal shunt catheters. Innov Neurosurg. 2013. 1: 169-72 11. Kazui H, Miyajima M, Mori E, Ishikawa M, SINPHONI-2 investigators. Lumboperitoneal shunt surgery for idiopathic normal pressure hydrocephalus (SINPHONI-2): An open-label randomised trial. Lancet Neurol. 2015. 14: 585-94 12. Kuwana N, Kuwabara T, Nakajima F, Hosoda H, Yamaguchi K. Simplified technique of lumbar subarachnoid-peritoneal shunt. No Shinkei Geka. 1977. 5: 229-34 13. Miyajima M, Kazui H, Mori E, Ishikawa M. One-year outcome in patients with idiopathic normal-pressure hydrocephalus: Comparison of lumboperitoneal shunt to ventriculoperitoneal shunt. J Neurosurg. 2016. 125: 1483-92 14. Miyake H. Shunt devices for the treatment of adult hydrocephalus: Recent progress and characteristics. Neurol Med Chir. 2016. 56: 274-83 15. Miyazaki T, Chiba A, Nishina H, Uesaka Y, Nakase H, Kanazawa I. Upper cervical myelopathy associated with low CSF pressure: A complication of ventriculoperitoneal shunt. Neurology. 1998. 50: 1864-6 16. Mokri B. Spontaneous low pressure, low CSF volume headaches: Spontaneous CSF leaks. Headache. 2013. 53: 1034-53 17. Mokri B. The Monro-Kellie hypothesis: Applications in CSF volume depletion. Neurology. 2001. 56: 1746-8 18. Mori E, Ishikawa M, Kato T, Kazui H, Miyake H.editors. Guidelines for management of idiopathic normal pressure hydrocephalus: Second edition. Neurol Med Chir. 2012. 52: 775-809 19. Nakajima M, Miyajima M, Akiba C, Ogino I, Kawamura K, Sugano H. Lumboperitoneal shunts for the treatment of idiopathic normal pressure hydrocephalus: A comparison of small-lumen abdominal catheters to gravitational add-on valves in a single center. Oper Neurosurg. 2018. 15: 634-42 20. Nakajima M, Miyajima M, Ogino I, Akiba C, Kawamura K, Kurosawa M. Shunt intervention for possible idiopathic normal pressure hydrocephalus improves patient outcomes: A nationwide hospital-based survey in Japan. Front Neurol. 2018. 9: 421 21. Podsiadlo D, Richardson S. The timed “Up and Go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991. 39: 142-8 22. Schievink WI, Maya MM, Louy C, Moser FG, Tourje J. Diagnostic criteria for spontaneous spinal CSF leaks and intracranial hypotension. AJNR Am J Neurorad. 2008. 29: 853-6 23. Tardieu GG, Fisahn C, Loukas M, Moisi M, Chapman J, Oskouian RJ. The epidural ligaments (of Hofmann): A comprehensive review of the literature. Cureus. 2016. 8: 779 24. Várallyay P, Nagy Z, Szűcs A, Czigléczki G, Markia B, Nagy G. Miyazaki syndrome: cervical myelo/radiculopathy caused by overshunting: A systematic review. Clin Neurol Neurosurg. 2019. 186: 105531 25. Wang VY, Barbaro NM, Lawton MT, Pitts L, Kunwar S, Parsa AT. Complications of lumboperitoneal shunts. Neurosurgery. 2007. 60: 1045-8 26. Watt S, Agerlin N, Romner B. Initial experience with the Codman Certas adjustable valve in the management of patients with hydrocephalus. Fluids Barriers CNS. 2012. 9: 21 27. Wnuk E, Maj E, Dziedzic T, Podlecka PA. Spinal epidural venous plexus enlargement as a cause of neurologic symptoms: Vascular anatomy and MRI findings. Neurol India. 2020. 68: 1238-41