- Department of Neurosurgery, Hospital de San José - Fundación Universitaria de Ciencias de la Salud, Bogota, Colombia,
- Department of Neuroradiology, The University of Texas MD Anderson Cancer Center, Houston, Texas, United States,
- Department of Neurological Surgery, Hospital de San José - Sociedad de Cirugía de Bogotá, Colombia
- Department of Diagnostic Imaging and Diagnostic Radiology, Fundación Universitaria de Ciencias de la Salud, Hospital Infantil Universitario de San José, Bogotá, Colombia,
- Department of Anatomy, University of Buenos Aires, Buenos Aires, Argentina,
- Department of Neurosurgery, Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado (ISSSTE), Mexico City, Mexico,
- Department of Neurosurgery, University of Pavia, Pavia, Italy,
- Department of Neurosurgery, Hospital Padilla de Tucuman, Tucuman, Argentina
- Department of Neurosurgery, San Fernando Hospital, San Fernando, Argentina.
Correspondence Address:
Matias Baldoncini, Department of Neurosurgery, San Fernando Hospital, San Fernando, Argentina.
DOI:10.25259/SNI_230_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Edgar G. Ordonez-Rubiano1, Jason M. Johnson2, Nadin Abdalá-Vargas3, Oscar F. Zorro1, Jorge H. Marin-Munoz1, Ricardo Álvarez-Tobián4, Valeria Forlizzi5, Carlos Castillo Rangel6, Sabino Luzzi7, Alvaro Campero8, Javier G. Patiño-Gómez1, Matias Baldoncini9. Preoperative tractography algorithm for safe resection of tumors located in the descending motor pathways zone. 21-Jul-2023;14:255
How to cite this URL: Edgar G. Ordonez-Rubiano1, Jason M. Johnson2, Nadin Abdalá-Vargas3, Oscar F. Zorro1, Jorge H. Marin-Munoz1, Ricardo Álvarez-Tobián4, Valeria Forlizzi5, Carlos Castillo Rangel6, Sabino Luzzi7, Alvaro Campero8, Javier G. Patiño-Gómez1, Matias Baldoncini9. Preoperative tractography algorithm for safe resection of tumors located in the descending motor pathways zone. 21-Jul-2023;14:255. Available from: https://surgicalneurologyint.com/surgicalint-articles/12450/
Abstract
Background: Diffusion tensor imaging (DTI) tractography facilitates maximal safe resection and optimizes planning to avoid injury during subcortical dissection along descending motor pathways (DMPs). We provide an affordable, safe, and timely algorithm for preoperative DTI motor reconstruction for gliomas adjacent to DMPs.
Methods: Preoperative DTI reconstructions were extracted from a prospectively acquired registry of glioma resections adjacent to DMPs. The surgeries were performed over a 7-year period. Demographic, clinical, and radiographic data were extracted from patients’ electronic medical records.
Results: Nineteen patients (12 male) underwent preoperative tractography between January 1, 2013, and May 31, 2020. The average age was 44.5 years (range, 19–81 years). A complete radiological resection was achieved in nine patients, a subtotal resection in five, a partial resection in three, and a biopsy in two. Histopathological diagnoses included 10 patients with high-grade glioma and nine with low-grade glioma. A total of 16 perirolandic locations (10 frontal and six frontoparietal) were recorded, as well as two in the insula and one in the basal ganglia. In 9 patients (47.3%), the lesion was in the dominant hemisphere. The median preoperative and postoperative Karnofsky Performance Scores were 78 and 80, respectively. Motor function was unchanged or improved over time in 15 cases (78.9%).
Conclusion: This protocol of DTI reconstruction for glioma removal near the DMP shows good results in low-term neurological functional outcomes.
Keywords: Brain tumor surgery, Diffusion tensor imaging, Neuro-oncology, Tractography, White matter
INTRODUCTION
The operative goal for an eloquently located glioma is to perform a maximal safe resection,[
Diffusion tensor imaging (DTI)-based tractography gives detailed information of the spatial relationship to functional boundaries of white matter connectivity between eloquent areas.[
In the early era of DTI-based tractography studies, multiple technical limitations (limited fiber tracts detailing, erroneous definition of fiber endings, contamination of fiber crossings, etc.) were described.[
We propose a descending motor pathway (DMP) fiber-tracking algorithm with clinically available software. This work aims to provide a timely and accessible protocol for tractography reconstruction in surgical planning for glioma resections near the DMP in order to minimize postoperative motor disability, while allowing a maximal safe resection.
MATERIALS AND METHODS
Patients
The senior authors reviewed a prospectively acquired database of patients who had consecutively undergone resection of gliomas adjacent to or involving the precentral gyrus (PrG) and/or the corticospinal tract (CST). Demographic, clinical, and imaging information were recorded for patients admitted to our two academic institutions between January 1, 2013, and May 31, 2020.
MRI was evaluated by the primary author (E.G.O.) in each patient to evaluate tumor location for study inclusion. Scans were reviewed by a dedicated neuroradiologist (J.M.J.) and one of the senior neurosurgeons for the confirmation of a location related to the DMPs. Fiber tracking was done by a dedicated neuroradiologist (J.M.J.) and assisted by a neurosurgeon (E.G.O.). The parameters that were quantified included a decrease in the volume of the tracts, displacement of fibers (fractional anisotropy [FA] values preserved), infiltration of the fibers (decreased FA values), and disruption of fibers (unable to detect continuity of the fibers), as we have described previously for language tracts.[
The clinical charts were assessed for demographic and clinical data. Extent of resection was calculated using tumor volume measurements obtained from MRI in three dimensions divided by a factor of two (A × B × C/2). The extent of removal was classified as gross total resection (100%), subtotal resection (≥90%), or partial resection (<90%). This study was performed according to the Declaration of Helsinki ethical standards. The Local Institutional Ethics Committee approved this study, and the manuscript was approved by the Institutional Review Board. The Institutional Ethics Committee considered that the informed consent was not required given the retrospective nature of this study and the research involves no more than minimal risk to the patients.
Imaging acquisition
Study data were acquired with a General Electric Signa Excite HDXT (1.5 T GE Healthcare, Milwaukee, WI, USA) and a Siemens MAGNETOM® Aera (1.5T Siemens Healthcare, Erlangen, Germany). An axial T1-weighted structural/anatomical and an axial DTI were acquired for each patient. Each structural image in T1 has 140 slices (1 mm thick, without GAP [free space], matrix = 320 × 192, repetition time [TR] = 650 ms, echo time [TE] = 22 ms, field of view [FOV] = 22, and acquisition time = 2 min and 35 s), covering the entire brain volume. For the isometric DTI sequence, a spin echo-planar imaging (EPI) sequence with 64 directions was used in an axial plane without angulation. Images were obtained from the base from the skull to the vertex. Each axial tensor sequence has 920 images, matrix = 100 × 100, TR = 14000 to 17000 ms, TE = minimum, thickness = 2.5 mm, spacing = 0.0, number of excitations (NEX) = 1, pixel = 2.5, FOV = 250, b value = 1000, and acquisition time = 7 min.
DTI fiber tracking
The manual selection of each region of interest (ROI) and the fiber tracking was performed with Functool 9.4.04b™ (by © General Electric Medical Systems) and Syngo DTI™ (by Siemens Healthcare). Correction of EPI distortions (scaling + translation + shearing) was applied. Volume rendering was used to overlay streamlines on the anatomical T1. Brainlab Elements SmartBrush and Fiber Tracking software tools (version 2.6) were used for neuronavigation imaging fusion of tractography. The ROIs were manually determined by recognition in the tensors in the three planes and T1 structural sequences. We used a Brain White Matter Atlas to define these ROIs.[
DMPs: CST and dorsal column-medial lemniscus (DCML)
For the reconstruction of the CST and the DCML, a single ROI was used in the anterior portion of the posterior limb of the internal capsule for each side. The posterior limb was chosen for this reconstruction as all DMPs are supposed to cross throughout this structure [
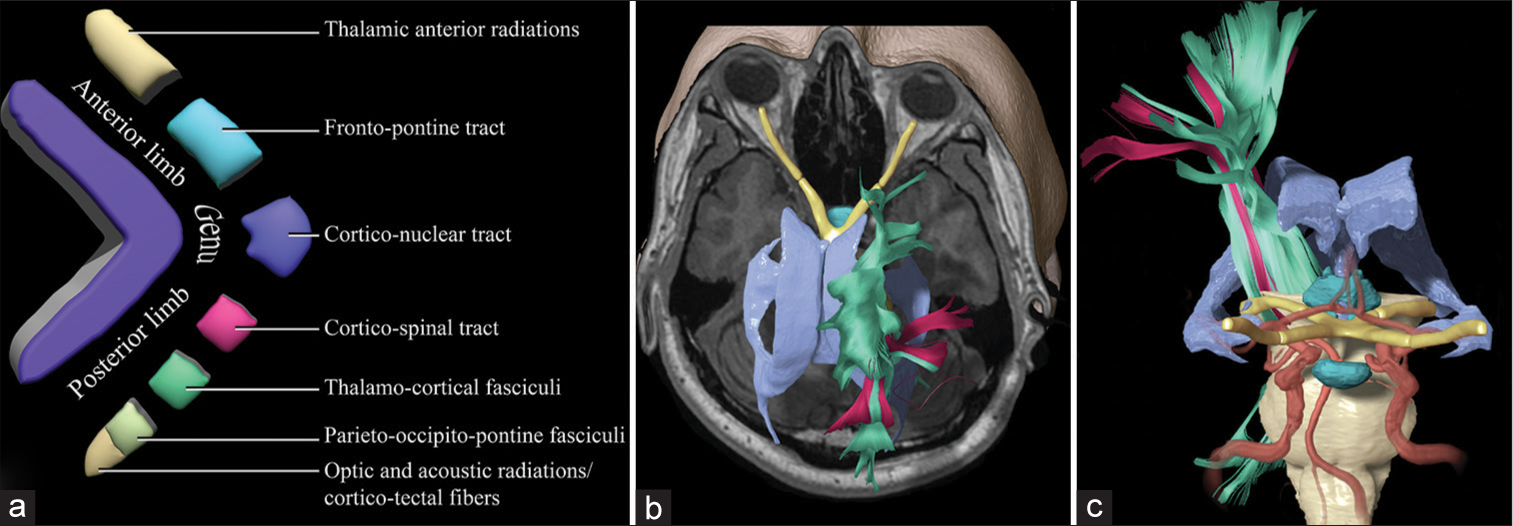
Figure 1:
Schematic of the internal capsule and the descending motor pathways. (a) Fragmented illustration of the components of the anterior limb, the genu, and the posterior limb. Tracts coming from the prefrontal and the supplementary motor areas descend through the anterior limb and the genu, including the thalamic anterior radiations, the frontopontine tracts, and the corticobulbar tracts, and the corticonuclear tracts. (b) Superior view of a three-dimensional reconstruction of the ventricles, the optic pathways, and the motor tracts. The tracts (green) that were reconstructed from a region of interest are located in the posterior limb of the internal capsule bilaterally, including both the corticospinal tract and the dorsal column-medial lemniscus. In pink are shown the tracts reconstructed from a region of interest in the precentral gyri bilaterally. (c) Anterior view of a three-dimensional reconstruction shows the anatomical relationships of the descending motor pathways with the ventricles, the hypothalamus, and the intracranial arterial system.
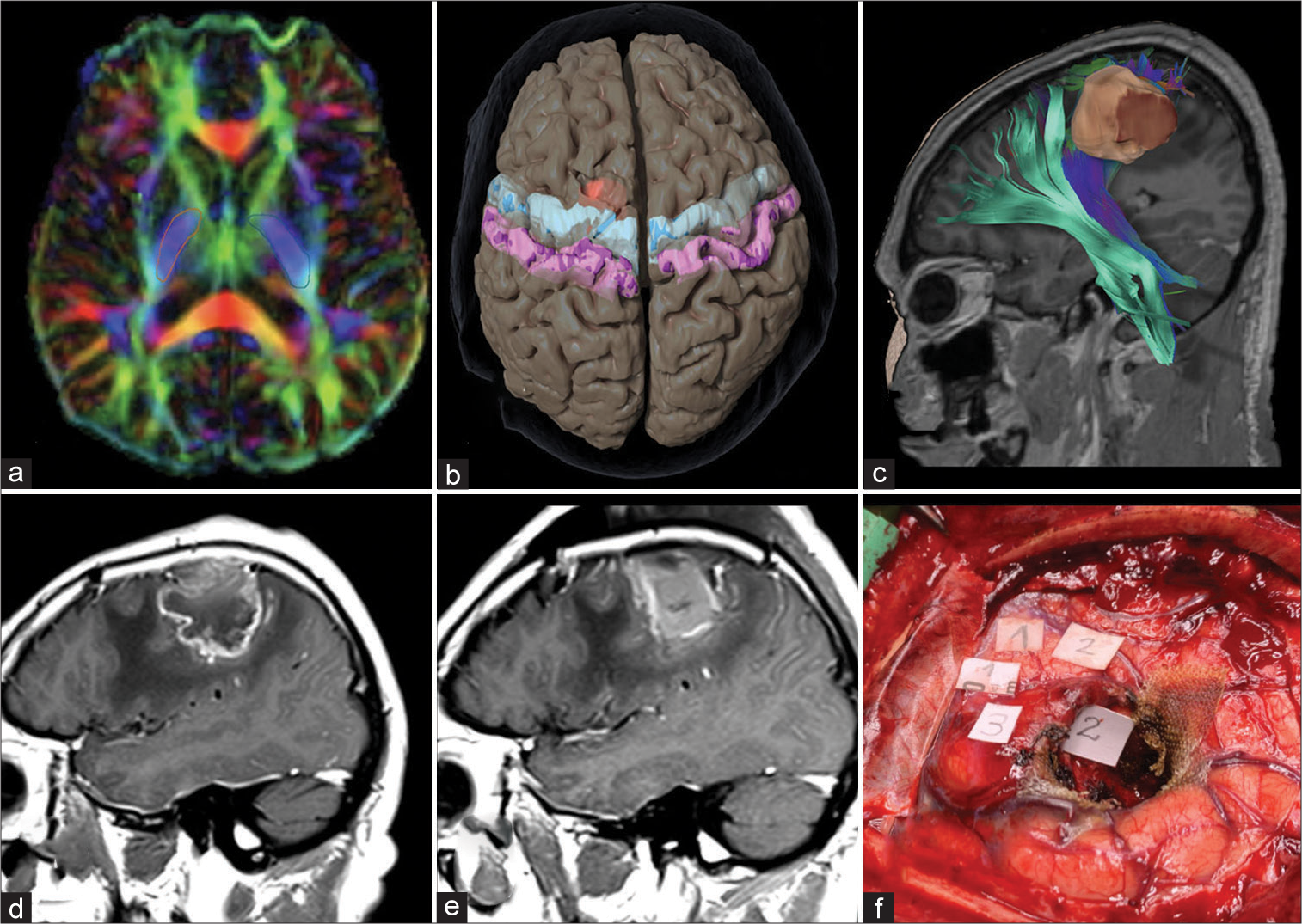
Figure 2:
Regions of interest used for fiber tracking. (a) Diffusion tensor imaging demonstrating selection of region of interests (ROIs) in the posterior limb of the internal capsule bilaterally. (b) The selection of ROIs in the precentral and postcentral gyri bilaterally is demonstrated. (c) Tractography reconstruction of descending motor pathway and the corticospinal tract in relationship with a left frontal tumor is shown. Preoperative (d) and postoperative (e) T1 postcontrast sagittal magnetic resonance imaging demonstrate a gross total resection of the tumor. (f) The intraoperative visualization of cortical and subcortical mapping with direct stimulation demonstrates the corresponding areas of the left shoulder (3), arm (2), and hand (1).
Surgical procedure
Patients were positioned in skull pin fixation for neuronavigation (Kolibri or Kick, BrainLab, Feldkirchen, Germany or Aimnav, Micromar Ind. Com. Ltda., Sao Paolo, Brazil). All surgical supplies and equipment were rented and were transferred to our institution the day before surgery, including the neuronavigation system, the physiological neuromonitoring system (NIM-Eclipse 4 System, Medtronic, Minneapolis, MN, USA) for asleep brain double motor mapping with monopolar and bipolar stimulation, and the ultrasonic aspirator (Sonoca, Söring, Germany). Induction of anesthesia was performed with propofol, and an initial neuromuscular block was performed with vecuronium or rocuronium to facilitate intubation as preferred by the anesthesiologist. Anesthesia was maintained with nitrous oxide/oxygen, supplemented by isoflurane and fentanyl or remifentanil in the majority of cases. Light surgical anesthesia was maintained, relying on opioid agents to provide adequate analgesia while minimally suppressing cortical responsiveness for motor mapping. Subdermal electrodes were used for electromyographic recording, motor evoked potentials, and sensory evoked potentials.
Skin incision and craniotomy were planned according to neuronavigation. If brain shift was observed after dural opening, an additional dose of 8 mg of dexamethasone was administered to revert millimetric displacement. When the tumor could be visualized, the motor cortex was stimulated to find a safe entry point. When the tumor was not visible at the cortical surface, a minimally invasive approach was performed after direct cortical stimulation (DCS). Total en bloc resection was performed when there was a subcortical cleavage plane; however, an internal debulking of the tumor was done in most patients. All efforts were made to preserve every cortical and subcortical vessel about the tumor to preserve adequate arterial supply and venous drainage of the primary motor cortex. Intermittent cortical and subcortical stimulations were used until achieving a maximal safe resection. At least two positive stimulations were required to consider an area as functional. High frequency stimulation was performed with a monopolar probe, with a train of 5 pulses, with 0.5 ms duration, and 1 Hz repetition rate. The intensity of the current used was 2–15 mA for the cortex, and 1–10 mA for the white matter. When a bipolar probe was used, the stimulus parameters were the same, but an increased current intensity was required. Threshold for stimulation was based on the electromyographic response to the electrical stimuli. Tumors were classified as low-grade glioma (LGG) (World Health Organization [WHO] Grade I or II glioma) and high-grade glioma (HGG) (WHO Grade III or IV glioma). Finally, closures were performed in a routine fashion.
RESULTS
The DMPs were detected bilaterally in all patients. In each patient, we identified DMPs coming from the motor cortex, from both the PrG and PoG, and DMPs passing through the posterior limb of the internal capsule, demonstrating fibers descending from the supplementary motor cortex and the prefrontal cortex delineating the corona radiata [
Twenty-five patients with gliomas were identified in which DTI fiber tracking and neuronavigation were used for surgical resection. Nineteen patients (12 males) aged 19–81 years (average 44.5 years) underwent resection of a glioma with use of preoperative fiber tracking of DMPs. Among these, 10 cases (52.6%) were HGGs, and 9 (47.4%) were LGGs. In nine patients, the lesion was in the dominant hemisphere. Eight patients (42.1%) presented with seizures, 8 (42.1%) with a motor deficit, 4 (21.0%) with a sensorial deficit, 6 (31.6%) with behavioral deficits, and 3 (15.8%) with both motor and language deficits. In 10 cases (52.6%), the lesion was in the frontal lobe, in 6 cases (31.6%) between the frontal and parietal lobes, in 2 cases (10.5%) in the insula, and in 1 case (5.3%) in the basal ganglia. Complete radiological removal was achieved in 9 cases (47.4%), subtotal resection in 5 (26.3%), partial resection in 3 (15.8%), and intended biopsies in 2 (10.5%). The fibers were infiltrated by the tumor in nine patients and displaced in 10 patients.
Assessment of motor function remained the same or improved in 15 cases (78.9%). A decline on the Karnofsky Performance Scale score directly associated with a postoperative motor deficit occurred in only two patients. One patient presented new-onset postoperative partial seizures, associated with an intended biopsy on an eloquent subcortical motor area after DCS. No other complications were observed. The mean follow-up time was 7 months (range, 1–12 months). Additional treatment with radiation only or radiation and chemotherapy was performed according to the indicated by the oncologist.
DISCUSSION
A new algorithm for DMPs reconstruction
Many authors have described preoperative reconstruction of DMP for resection of tumors associated with these tracts worldwide in the past 20 years, since the beginning of this technology.[
In this study, we propose to include information according to tumor location. If possible, primary motor cortex (perirolandic) tumors should be evaluated with both DTI and blood oxygen level-dependent acquisitions. Information from both should be explored and processed to be included in neuronavigation planning. For these tumors, the tractography reconstruction should be performed bilaterally including the following tracts separately: One ROI at the posterior limb of the internal capsule including both the CST and the DCML, an additional ROI starting from the activated areas with the motor fMRI and completing information with a cortical ROI including both PrG and PoG. It is important to note that for perirolandic tumors, DCS and DSS represent the gold standard, and this tractography protocol may serve as a road map for stimulation, giving information for the delineation of the most critical areas to be stimulated. In addition, the relation of the tracts (anterior, posterior, medial, or lateral) with the tumor confirms the correct position of the CST, preventing an unexpected injury. To avoid postoperative supplementary motor area syndrome in tumors located anteriorly in the supplementary motor area, we recommend reconstructing additional peritumoral U fibers to prevent a postoperative supplementary motor area syndrome.[
To incorporate the protocol discussed in this study for preoperative DTI reconstruction for glioma resection near the DMP, our goal is to simplify tools, including advanced preoperative imaging, which may be available but often neglected by surgeons, facilitating maximal safe resection. We present an easy way to reproduce all motor tracts with commercially and available software, using corresponding seeding points for ROIs to identify possible entry points and trajectories to motor areas related to gliomas. This surgical planning allows surgeons to identify routes without damaging motor tracts. We introduce a combination of ROIs to identify all DMPs, which could be represented as a combination of eloquent areas including the posterior limb of the internal capsule, peritumoral U fibers, the PrG, and the PoG, which can be used as anatomical landmarks. Displacement of the DMP makes a path for surgeons for a safe intraparenchymal corridor.
Intraoperative mapping with direct cortical and subcortical stimulation cannot be substituted with preoperative tractography and remains the gold standard for determining motor function in glioma resection.[
Illustrative case
A 36-year-old woman presented with complex seizures. Her antecedents and physical examination were unremarkable. Preoperative contrast-enhanced MRI demonstrated a right frontal tumor, with minimal enhancement, most consistent with an LGG. The patient participated in a preoperative discussion and agreed that if the tumor could not be resected without producing a permanent motor deficit, then only a biopsy would be performed. Intraoperatively, multiple responses were obtained from the majority of the tumor area [
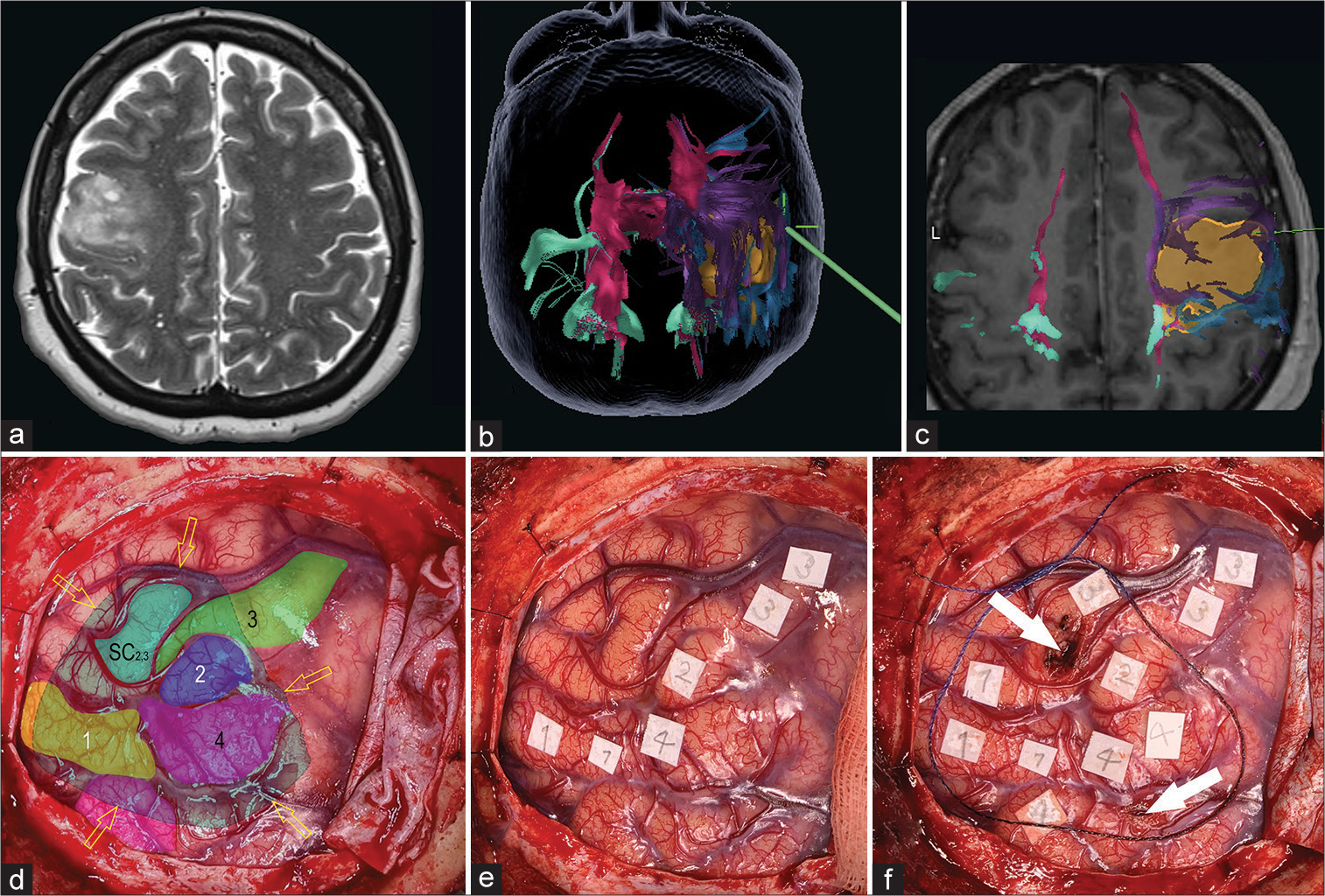
Figure 3:
Preoperative magnetic resonance imaging, neuronavigation fused tractography, and intraoperative images of an intentional biopsy. (a) Axial T2 image reveals a right frontal heterogeneous hyperintense intra-axial lesion. (b and c) Neuronavigation images of tractography of descending motor pathways. The volumetric reconstruction of the tumor is shown in yellow. Also shown are the motor tracts coming from the posterior limb of the internal capsule (pink), from the supplementary area (purple), and from the medial (green) and lateral (blue) precentral gyrus. (d-f) Intraoperative images of brain mapping with direct cortical stimulation. Numbers 1 (yellow), 2 (dark blue), 3 (green), and 4 (pink) show the motor areas of the tongue, eye, upper limb, and mouth, respectively. Yellow arrows show the borders of the tumor area verified with the neuronavigation system; white arrows show the safest areas of the cortex from which the biopsy was taken. The aquamarine area (SC2,3) shows where a subcortical response from the eye and the upper limb was noted during subcortical mapping during the biopsy.
Study limitations
This retrospective study was limited by the quality of data collected within the medical charts and by the small number of cases in whom the tractography was performed. Examination by an independent examiner may have revealed subtle deficits that could be missed during follow-up, and documentation of motor outcome could be biased. Whereas the tractography is a unique tool that permits an individual analysis of the white matter for each patient, the DTI reconstruction also remains with the limitations mentioned above. This study lacks information comparing tractography and DSS; however, fiber tracking was used to improve subcortical stimulation.
CONCLUSION
Separate reconstructions of tracts from all DMPs can be performed preoperatively in a timely and accurate manner to improve brain mapping for resection of tumors associated with motor areas, consequently helping surgeons preserve the patients’ motor function. In addition, this reconstruction, when fused with neuronavigation, may serve as a road map during resection for subcortical stimulation. However, additional studies are needed to compare the accuracy of information between pre and postoperative tractography as well as between tractography and DSS.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Alexander DC, Barker GJ. Optimal imaging parameters for fiber-orientation estimation in diffusion MRI. Neuroimage. 2005. 27: 357-67
2. Alexopoulos G, Cikla U, El Tecle N, Kulkarni N, Pierson M, Mercier P. The value of white matter tractography by diffusion tensor imaging in altering a neurosurgeon’s operative plan. World Neurosurg. 2019. 132: e305-13
3. Berman JI, Chung S, Mukherjee P, Hess CP, Han ET, Henry RG. Probabilistic streamline q-ball tractography using the residual bootstrap. Neuroimage. 2008. 39: 215-22
4. Bozkurt B, Yagmurlu K, Middlebrooks EH, Cayci Z, Cevik OM, Karadag A. Fiber connections of the supplementary motor area revisited: Methodology of fiber dissection, DTI, and three dimensional documentation. J Vis Exp. 2017. 123: 55681
5. Bucci M, Mandelli ML, Berman JI, Amirbekian B, Nguyen C, Berger MS. Quantifying diffusion MRI tractography of the corticospinal tract in brain tumors with deterministic and probabilistic methods. Neuroimage Clin. 2013. 3: 361-8
6. Bulubas L, Sollmann N, Tanigawa N, Zimmer C, Meyer B, Krieg SM. Reorganization of motor representations in patients with brain lesions: A navigated transcranial magnetic stimulation study. Brain Topogr. 2018. 31: 288-99
7. Caverzasi E, Hervey-Jumper SL, Jordan KM, Lobach IV, Li J, Panara V. Identifying preoperative language tracts and predicting postoperative functional recovery using HARDI q-ball fiber tractography in patients with gliomas. J Neurosurg. 2016. 125: 33-45
8. Cho ZH, Calamante F, Chi JG, editors. 7.0 Tesla MRI Brain White Matter Atlas. New York, NY: Springer; 2015. p.
9. Claus EB, Walsh KM, Wiencke JK, Molinaro AM, Wiemels JL, Schildkraut JM. Survival and low-grade glioma: The emergence of genetic information. Neurosurg Focus. 2015. 38: E6
10. De Witt Hamer PC, Robles SG, Zwinderman AH, Duffau H, Berger MS. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: A meta-analysis. J Clin Oncol. 2012. 30: 2559-65
11. Duffau H, Gatignol P, Mandonnet E, Peruzzi P, TzourioMazoyer N, Capelle L. New insights into the anatomo-functional connectivity of the semantic system: A study using cortico-subcortical electrostimulations. Brain. 2005. 128: 797-810
12. Fernandez-Miranda JC, Pathak S, Schneider W. High-definition fiber tractography and language. J Neurosurg. 2010. 113: 156-57 author reply 157-8
13. Fernandez-Miranda JC, Rhoton AL, Alvarez-Linera J, Kakizawa Y, Choi C, de Oliveira EP. Three-dimensional microsurgical and tractographic anatomy of the white matter of the human brain. Neurosurgery. 2008. 62: 989-1026 discussion 1026-8
14. Florman JE, Duffau H, Rughani AI. Lower motor neuron findings after upper motor neuron injury: Insights from postoperative supplementary motor area syndrome. Front Hum Neurosci. 2013. 7: 85
15. Garrett MC, Pouratian N, Liau LM. Use of language mapping to aid in resection of gliomas in eloquent brain regions. Neurosurg Clin N Am. 2012. 23: 497-506
16. Ghinda D, Zhang N, Lu J, Yao CJ, Yuan S, Wu JS. Contribution of combined intraoperative electrophysiological investigation with 3-T intraoperative MRI for awake cerebral glioma surgery: Comprehensive review of the clinical implications and radiological outcomes. Neurosurg Focus. 2016. 40: E14
17. Ginat DT, Swearingen B, Curry W, Cahill D, Madsen J, Schaefer PW. 3 Tesla intraoperative MRI for brain tumor surgery. J Magn Reson Imaging. 2014. 39: 1357-65
18. Gogos AJ, Young JS, Morshed RA, Avalos LN, Noss RS, Villanueva-Meyer JE. Triple motor mapping: Transcranial, bipolar, and monopolar mapping for supratentorial glioma resection adjacent to motor pathways. J Neurosurg. 2020. 134: 1728-37
19. Gossl C, Fahrmeir L, Putz B, Auer LM, Auer DP. Fiber tracking from DTI using linear state space models: Detectability of the pyramidal tract. Neuroimage. 2002. 16: 378-88
20. Guye M, Parker GJ, Symms M, Boulby P, Wheeler-Kingshott CA, Salek-Haddadi A. Combined functional MRI and tractography to demonstrate the connectivity of the human primary motor cortex in vivo. Neuroimage. 2003. 19: 1349-60
21. Hamidian S, Vachha B, Jenabi M, Karimi S, Young RJ, Holodny AI. Resting-state functional magnetic resonance imaging and probabilistic diffusion tensor imaging demonstrate that the greatest functional and structural connectivity in the hand motor homunculus occurs in the area of the thumb. Brain Connect. 2018. 8: 371-9
22. Henry RG, Berman JI, Nagarajan SS, Mukherjee P, Berger MS. Subcortical pathways serving cortical language sites: Initial experience with diffusion tensor imaging fiber tracking combined with intraoperative language mapping. Neuroimage. 2004. 21: 616-22
23. Hollon T, Lewis S, Freudiger CW, Xie XS, Orringer DA. Improving the accuracy of brain tumor surgery via Raman-based technology. Neurosurg Focus. 2016. 40: E9
24. Jenabi M, Peck KK, Young RJ, Brennan N, Holodny AI. Identification of the corticobulbar tracts of the tongue and face using deterministic and probabilistic DTI fiber tracking in patients with brain tumor. AJNR Am J Neuroradiol. 2015. 36: 2036-41
25. Leclercq D, Delmaire C, de Champfleur NM, Chiras J, Lehericy S. Diffusion tractography: Methods, validation and applications in patients with neurosurgical lesions. Neurosurg Clin N Am. 2011. 22: 253-68
26. Majchrzak K, Bobek-Billewicz B, Hebda A, Adamczyk P, Majchrzak H, Ladzinski P. Surgical treatment of adult patients with thalamic tumors with the aid of tractography, fMRI, transcranial electrical stimulation and direct electrical stimulation of the subcortical white matter. Neurol Neurochir Pol. 2018. 52: 720-30
27. Mandelli ML, Berger MS, Bucci M, Berman JI, Amirbekian B, Henry RG. Quantifying accuracy and precision of diffusion MR tractography of the corticospinal tract in brain tumors. J Neurosurg. 2014. 121: 349-58
28. McGirt MJ, Mukherjee D, Chaichana KL, Than KD, Weingart JD, Quinones-Hinojosa A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009. 65: 463-69 discussion 469-70
29. Mitchell TJ, Hacker CD, Breshears JD, Szrama NP, Sharma M, Bundy DT. A novel data-driven approach to preoperative mapping of functional cortex using resting-state functional magnetic resonance imaging. Neurosurgery. 2013. 73: 969-82 discussion 982-63
30. Moritz-Gasser S, Herbet G, Maldonado IL, Duffau H. Lexical access speed is significantly correlated with the return to professional activities after awake surgery for low-grade gliomas. J Neurooncol. 2012. 107: 633-41
31. Munnich T, Klein J, Hattingen E, Noack A, Herrmann E, Seifert V. Tractography verified by intraoperative magnetic resonance imaging and subcortical stimulation during tumor resection near the corticospinal tract. Oper Neurosurg (Hagerstown). 2019. 16: 197-210
32. Negwer C, Sollmann N, Ille S, Hauck T, Maurer S, Kirschke JS. Language pathway tracking: Comparing nTMS-based DTI fiber tracking with a cubic ROIs-based protocol. J Neurosurg. 2016. 126: 1006-14
33. Nimsky C, Ganslandt O, Hastreiter P, Wang R, Benner T, Sorensen AG. Intraoperative diffusion-tensor MR imaging: Shifting of white matter tracts during neurosurgical procedures--initial experience. Radiology. 2005. 234: 218-25
34. Ordonez-Rubiano EG. 3D microsurgical anatomy of the corticospinal tract and the lemniscus tract based on microdissection of fibres and demonstration through tractography. Neurocirugia (Astur: Eng Ed). 2019. 30: 309-10
35. Ordonez-Rubiano EG, Valderrama-Arias FA, Forbes JA, Johnson JM, Younus I, Marin-Munoz JH. Identification of preoperative language tracts for intrinsic frontotemporal diseases: A pilot reconstruction algorithm in a middle-income country. World Neurosurg. 2019. 125: e729-42
36. Parra-Morales AM, Rudas J, Vargas JA, Gomez F, Enciso-Olivera CO, Trujillo-Rodriguez D. Structural and functional connectivity of ascending reticular activating system in a patient with impaired consciousness after a cardiac arrest: A case report. Medicine (Baltimore). 2019. 98: e15620
37. Radmanesh A, Zamani AA, Whalen S, Tie Y, Suarez RO, Golby AJ. Comparison of seeding methods for visualization of the corticospinal tracts using single tensor tractography. Clin Neurol Neurosurg. 2015. 129: 44-9
38. Raffa G, Conti A, Scibilia A, Sindorio C, Quattropani MC, Visocchi M. Functional reconstruction of motor and language pathways based on navigated transcranial magnetic stimulation and DTI fiber tracking for the preoperative planning of low grade glioma surgery: A new tool for preservation and restoration of eloquent networks. Acta Neurochir Suppl. 2017. 124: 251-61
39. Sanai N, Polley MY, Berger MS. Insular glioma resection: Assessment of patient morbidity, survival, and tumor progression. J Neurosurg. 2010. 112: 1-9
40. Schonberg T, Pianka P, Hendler T, Pasternak O, Assaf Y. Characterization of displaced white matter by brain tumors using combined DTI and fMRI. Neuroimage. 2006. 30: 1100-11
41. Sollmann N, Negwer C, Tussis L, Hauck T, Ille S, Maurer S. Interhemispheric connectivity revealed by diffusion tensor imaging fiber tracking derived from navigated transcranial magnetic stimulation maps as a sign of language function at risk in patients with brain tumors. J Neurosurg. 2017. 126: 222-33
42. Szmuda T, Kieronska S, Ali S, Sloniewski P, Pacholski M, Dzierzanowski J. Tractography-guided surgery of brain tumors: What is the best method to outline the corticospinal tract?. Folia Morphol (Warsz). 2020. 80: 40-6
43. Teixidor P, Gatignol P, Leroy M, Masuet-Aumatell C, Capelle L, Duffau H. Assessment of verbal working memory before and after surgery for low-grade glioma. J Neurooncol. 2007. 81: 305-13
44. Wedeen VJ, Hagmann P, Tseng WY, Reese TG, Weisskoff RM. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Reson Med. 2005. 54: 1377-86
45. Weiss C, Tursunova I, Neuschmelting V, Lockau H, Nettekoven C, Oros-Peusquens AM. Improved nTMS-and DTI-derived CST tractography through anatomical ROI seeding on anterior pontine level compared to internal capsule. Neuroimage Clin. 2015. 7: 424-37
46. Wu JS, Zhou LF, Tang WJ, Mao Y, Hu J, Song YY. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: A prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery. 2007. 61: 935-48 discussion 948-39
47. Yu Q, Lin K, Liu Y, Li X. Clinical uses of diffusion tensor imaging fiber tracking merged neuronavigation with lesions adjacent to corticospinal tract: A retrospective cohort study. J Korean Neurosurg Soc. 2020. 63: 248-60
48. Zakaria H, Haider S, Lee I. Automated whole brain tractography affects preoperative surgical decision making. Cureus. 2017. 9: e1656
49. Zarabi H, Roy A, Jha A, Pradilla G. Refining surgical corridors with whole brain tractography: A case series. Cureus. 2017. 9: e1672
50. Zhu F, Zhuang D, Luo Q, Qiu T, Wu J, Feng J. 208 Connectivity-based functional parcellation and localization of the human supplementary motor area based on resting-state functional magnetic resting imaging and its utility in brain tumor surgery. Neurosurgery. 2016. 63: 181-2