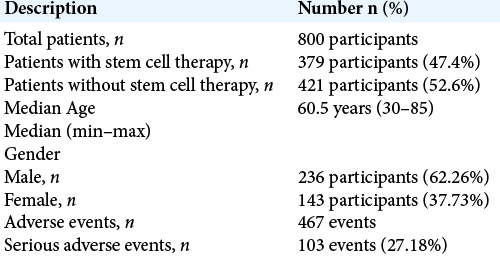- Department of Neurosurgery Faculty of Medicine, Universitas Airlangga/Dr. Soetomo General Academic Hospital, Surabaya, East Java, Indonesia.
- Department of Public Health, Faculty of Medicine, Universitas Airlangga/Dr. Soetomo General Academic Hospital, Surabaya, East Java, Indonesia.
Correspondence Address:
Asra Al Fauzi, Departments of Neurosurgery, Faculty of Medicine, Universitas Airlangga/Dr. Soetomo General Academic Hospital, Surabaya, East Java, Indonesia.
DOI:10.25259/SNI_1174_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Andhika Tomy Permana1, Abdul Hafid Bajamal1, Muhammad Arifin Parenrengi1, Nur Setiawan Suroto1, Pudji Lestari2, Asra Al Fauzi1. Clinical outcome and safety of stem cell therapy for ischemic stroke: A systematic review and meta-analysis. 20-May-2022;13:206
How to cite this URL: Andhika Tomy Permana1, Abdul Hafid Bajamal1, Muhammad Arifin Parenrengi1, Nur Setiawan Suroto1, Pudji Lestari2, Asra Al Fauzi1. Clinical outcome and safety of stem cell therapy for ischemic stroke: A systematic review and meta-analysis. 20-May-2022;13:206. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11610
Abstract
Background: Several reports on stem cell administration have emerged proving it to be an ideal therapeutic approach for improving neurological functions in ischemic stroke patients. However, some studies also show disappointing results, with some reporting no statistically significant improvements among several different parameters. Several challenges also arise relating to safety and nonscientific aspects, such as ethics.
Methods: We performed a systematic review and meta-analysis to evaluate the effect of stem cell therapy on the clinical outcomes of ischemic stroke patients. A systematic review and meta-analysis were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. A thorough literature search was conducted on PubMed, Scopus, and Cochrane databases. Articles were selected systematically based on the PRISMA protocol and reviewed completely. A total of 19 publications pertaining to stem cell therapy on the ischemic route were included and reviewed. Efficacy outcomes were measured with the National Institutes of Health Stroke Scale, modified Rankin Scale, or Barthel Index.
Results: The results of the meta-analysis indicate that the efficacy outcomes suggest favorable results after stem cell therapy, although not all study results are statistically significant. Stem cell therapy in stroke cases showed a better outcome than standard conservative therapy alone, although our analysis shows that many factors can influence this outcome, and significant effects can only be seen after several months.
Conclusion: The results of this study show promising and satisfying efficacy and a relatively low rate of serious adverse events.
Keywords: Clinical outcome, Ischemic stroke, Safety, Stem cell therapy
INTRODUCTION
Ischemic stroke is the main subtype of stroke and occurs in about 70% of all stroke cases (85– 87% in the United States),[
Due to these disabilities, standard interventions (including intravenous thrombolysis and mechanical endovascular clot retrieval) have been shown to improve outcomes, including survival, and residual disability.[
Intravenous recombinant tissue plasminogen activator, currently the only approved substance for ischemic stroke intervention, has a narrow efficacy time window of only 4.5 h, and reportedly only up to 5% of patients are able to receive this therapy.[
Recently, reports of stem cell administration have emerged, suggesting that it is an ideal therapeutic approach for improving neurological functions in ischemic stroke patients.[
Animal studies have proven that various types of stem cells are able to improve neurological functions that occur after cerebral stroke.[
We performed a systematic review and meta-analysis to evaluate clinical outcomes of ischemic stroke patients after stem cell therapy. To explore any future hypotheses, it was important to undertake this systematic review to obtain basic information and data on the efficacy of stem cell therapy for ischemic stroke cases. It is also necessary to help clinicians and stakeholders in the decision-making process to determine whether stem cell therapy for ischemic stroke patients needs to be promoted, and to determine its benefits and associated challenges.
MATERIALS AND METHODS
Eligibility criteria
There was a full-text cohort study including clinical trials on adult patients (>18-years-old) with ischemic stroke, in any phase of the disease (acute, subacute, or chronic), who received stem cell therapy with intracerebral, intraventricular, subarachnoid, intra-arterial, intravenous, intraperitoneal, or intranasal administration. Reviews, unpublished articles, letters to the editor, abstracts, and studies not written in English were excluded from the study.
Type of outcome measures
Clinical outcome measured with:
Modified Rankin Scale (mRS) National Institute of Health Stroke Scale (NIHSS) Barthel Index (BI)
We also assessed that the safety of stem cell therapy outlined in these studies by determining the number and severity of any adverse events.
Information sources
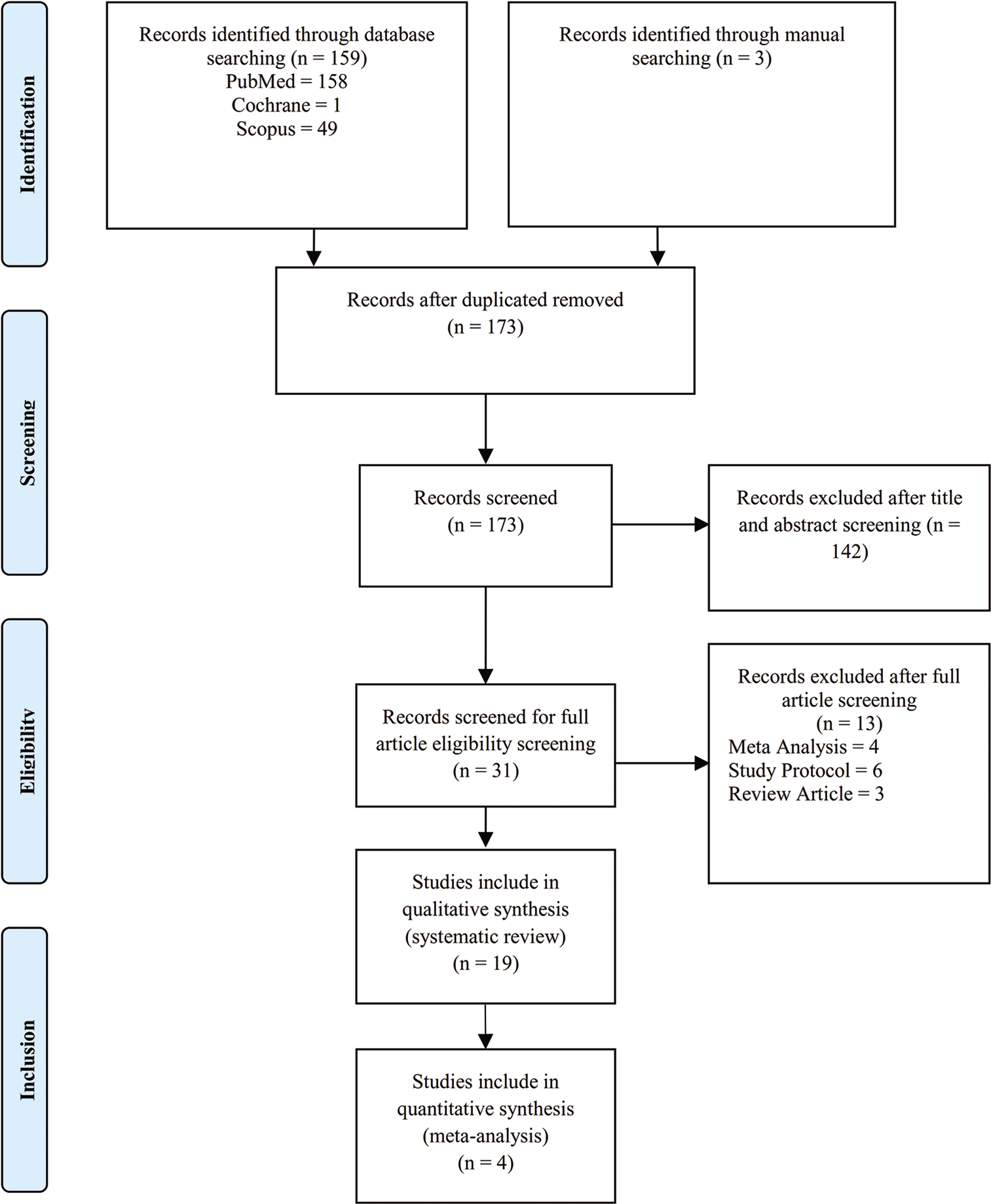
This systematic review was conducted based on Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines as shown in [
Search protocol
The study question was formed using the patient/population, intervention, comparison, and outcomes model. The authors used the following search keywords to search all trials registers and databases: stem cells therapy AND (ischemic stroke OR ischemic brain) AND (mRS OR NIHSS OR BI).
Data collection and analysis
We screened all records by the title and abstract as our search strategy. Three authors (ATP, PL, and AAF) independently assessed the inclusion of all potential studies. The search results were first excluded based on the relevancy of the titles and then on the relevancy of the abstracts. Non English publications were automatically excluded from the study. Full-text articles were then assessed by all authors for potentially eligible randomized and controlled trials (RCTs) and cohort studies. The reasons for exclusion of studies were noted and reported. Included studies are shown in [
Data extraction and management
For eligible studies, three review authors (HF, MAF, and NSS) independently extracted data using the data extraction form on characteristics of patients and interventions, study quality, and outcomes of interventions.[
Assessment of study quality and risk of bias in included studies
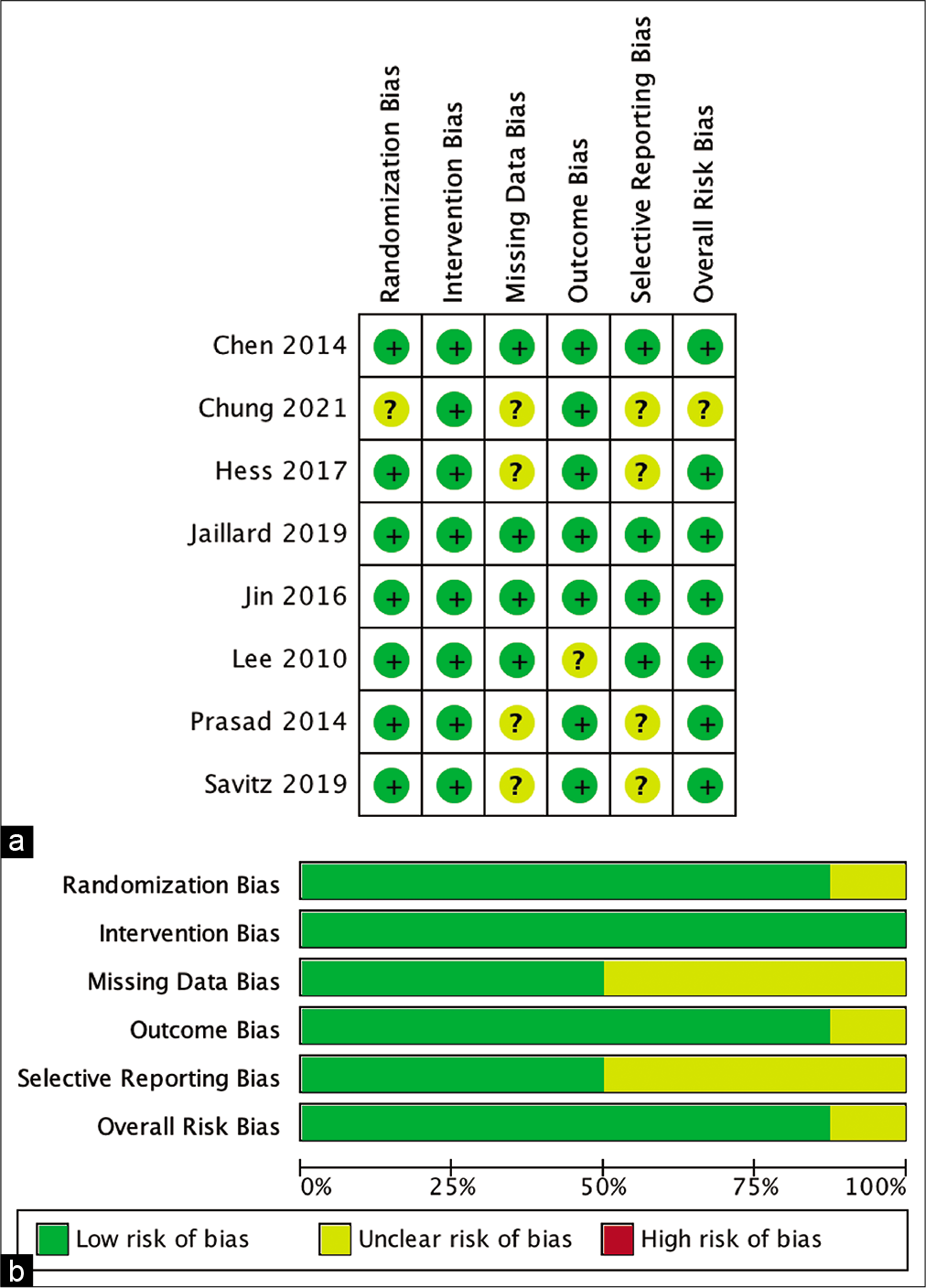
The review authors independently assessed risk of bias for each included study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions for nonrandomized studies: Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I) for nonrandomized studies and Risk of Bias 2 (RoB 2) for randomized studies.[
Measures of treatment effect
We presented the results of the continuous data as mean difference with 95% CI to combine trials that measured the same outcome and same comparison. A meta-analysis was planned if the data were appropriate for pooling. Summary of estimates was presented as mean difference for the outcomes. Consequently, both fixed-effects and random-effects meta-analyses were used, although the latter was prespecified in the protocol. If pooling was inappropriate, a narrative synthesis was implemented. The definitions of CI, heterogeneity, and P-value were conventional (CI 95%; I2 <40%: unimportant; 30–60%: moderate; 50–90%: substantial, 75–100%: considerable; P < 0.05: significant; and P value for interaction < 0.1: significant).[
RESULTS
A total of 173 studies were identified and screened. Of these, 31 studies were assessed for eligibility, 19 studies were included in the qualitative review,[
Demographic results
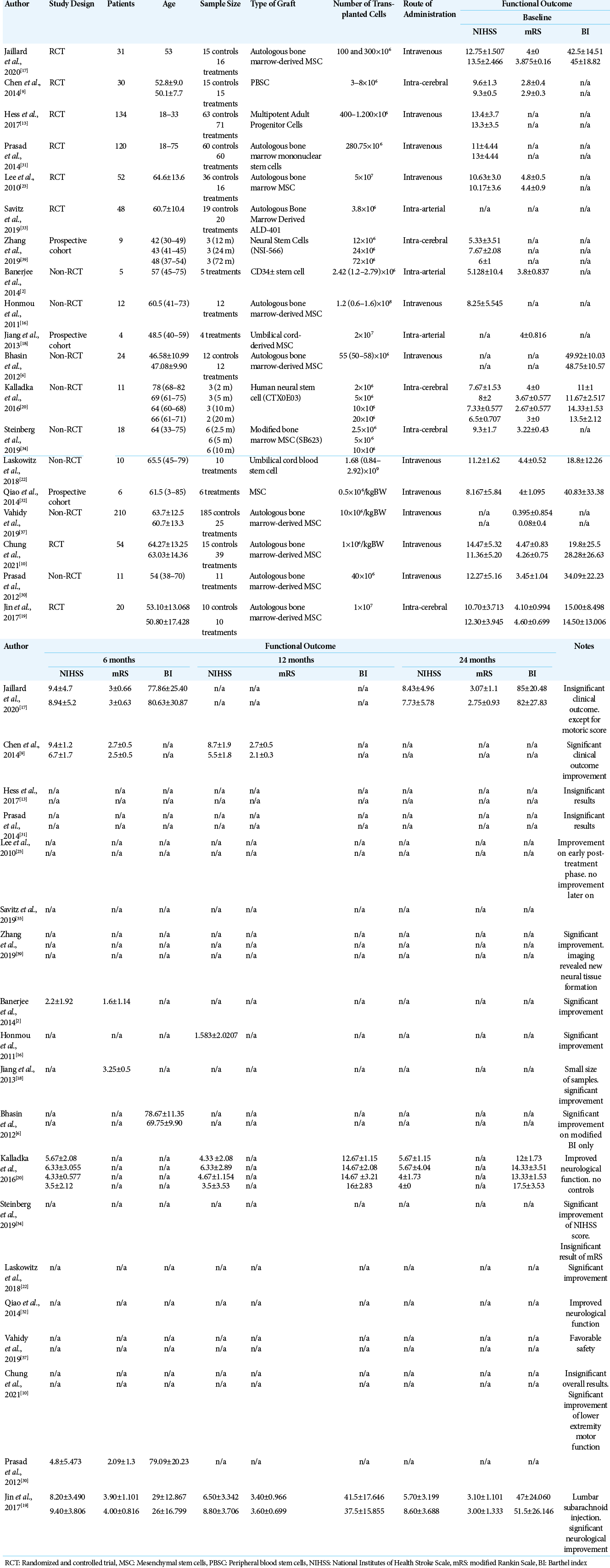
The 19 studies in the review included 800 patients with a median age of 60.5 years (range 30–85 years). Male participants dominated in the study, comprising 236 (62.26%) of the participants versus 143 (37.73%) females.
The patients were then divided into two groups: an experimental/ therapy group (379 patients) and a control group who did not receive stem cell therapy administration (421 patients). Adverse events were reported 467 times in 19 different studies. Of those 467 documented adverse events, 103 (27.18%) were serious. A summary of the results is shown in [
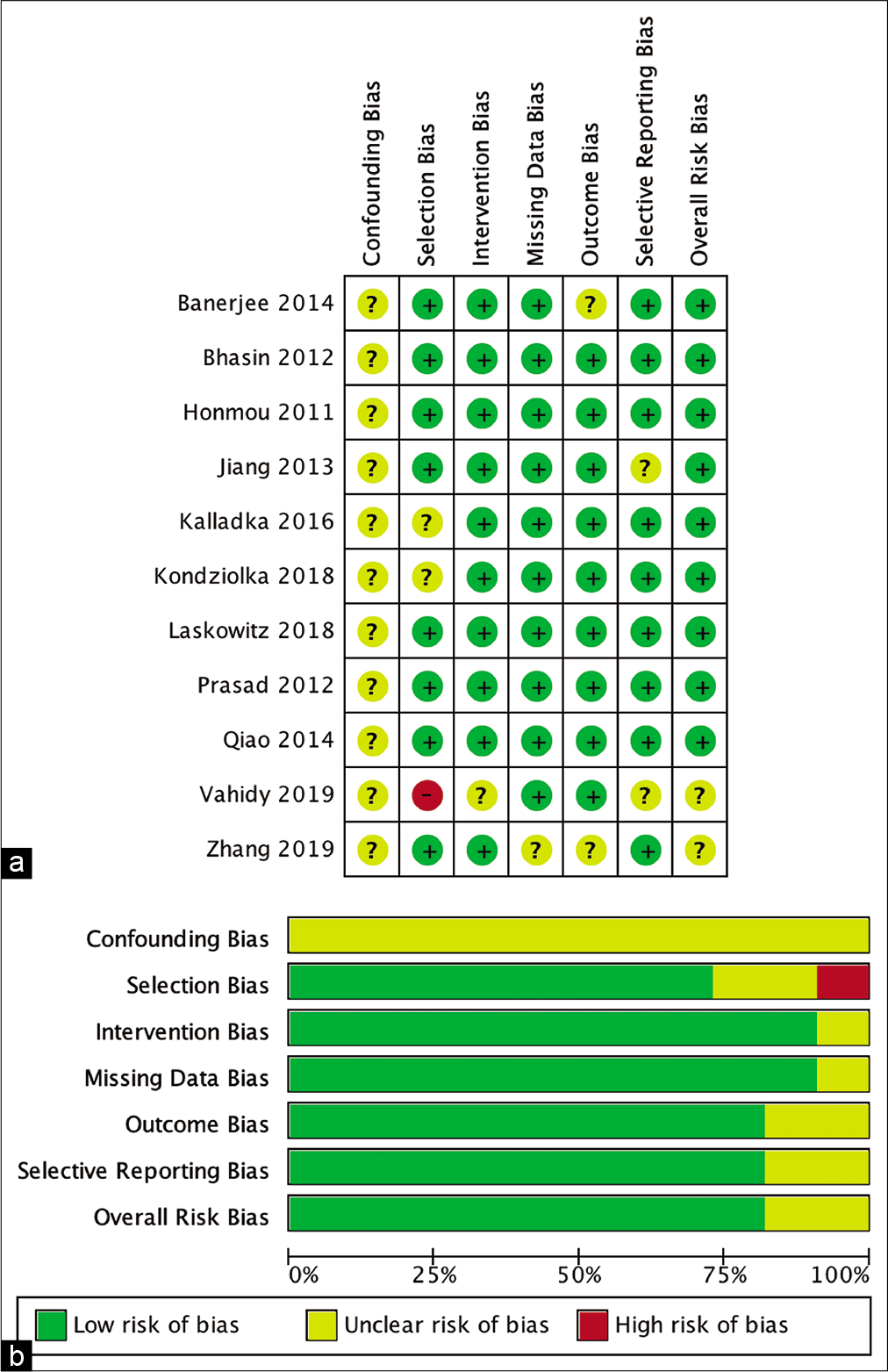
Risk of bias analysis
The risk of bias risk assessment of the studies involved was measured by ROBINS-I for nonrandomized studies and the RoB 2 tool. The result is shown in [
Stem cell versus control group outcome comparison
Out of all the 19 studies included in this review, 4 (21.05%) studies were able to be included in the quantitative analysis for the 6-, 12-, and 24-months posttherapy neurological outcomes measured in NIHSS, mRS, and BI, respectively. The comparison was undertaken between those who received and those who did not receive stem cell therapy at the time when the baseline neurological functions were measured.
6-month outcome
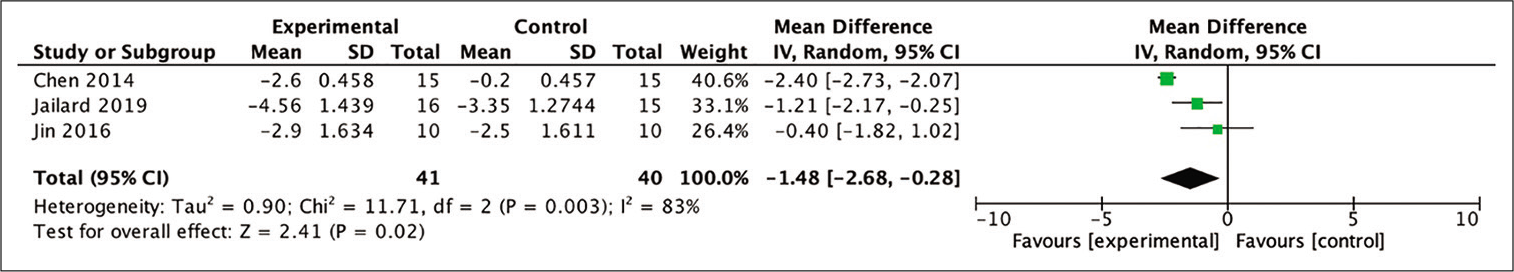
6-month NIHSS
Out of the four studies included in the meta-analysis, three were eligible to be included in the analysis for the improvement in the NIHSS score by calculating the mean difference between the baseline and 6-month posttherapy scores. The assessment was carried out to assess the difference in mean NIHSS scores in the stem cell therapy and control groups after 6 months. The results showed a favorable trend in the stem cell therapy group and these results were statistically significant (MD = 1.48; 95% CI −2.68–−0.28; P = 0.02; and I2 = 83%). The results of the complete analysis, including a diagram, are shown in [
Figure 4:
Calculation of the mean difference in NIHSS scores after 6 months between stem cell therapy and control groups in three studies. The mean difference was statistically greater in the stem cell therapy group and this result was statistically significant (MD = −1.48; 95% CI −2.68–−0.28; P = 0.02; and I2 = 83%).
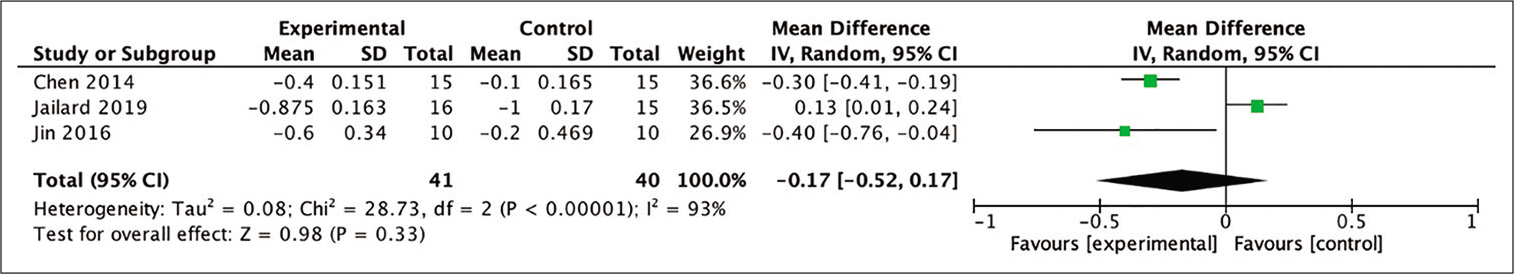
6-month mRS
In the analysis of improvement in mRS scores, three out of four studies included in the meta-analysis provided the necessary data so that the mean difference between baseline and 6-month posttherapy scores could be calculated. The assessment evaluated the mean difference in mRS scores in the stem cell therapy and control groups after 6 months of observation. Despite not being statistically significant, the difference between two groups indicated a more favorable result in patients who received stem cell therapy (MD = −0.27; 95% CI −0.52–0.17; P = 0.33; and I2 = 93%), as shown in [
Figure 5:
Calculation of the mean difference in mRS scores after 6 months between the stem cell therapy and control groups in three studies. The mean difference was statistically greater in the stem cell therapy group, although this result was not statistically significant (MD = −0.27; 95% CI −0.52–0.17; P = 0.33; and I2 = 93%).
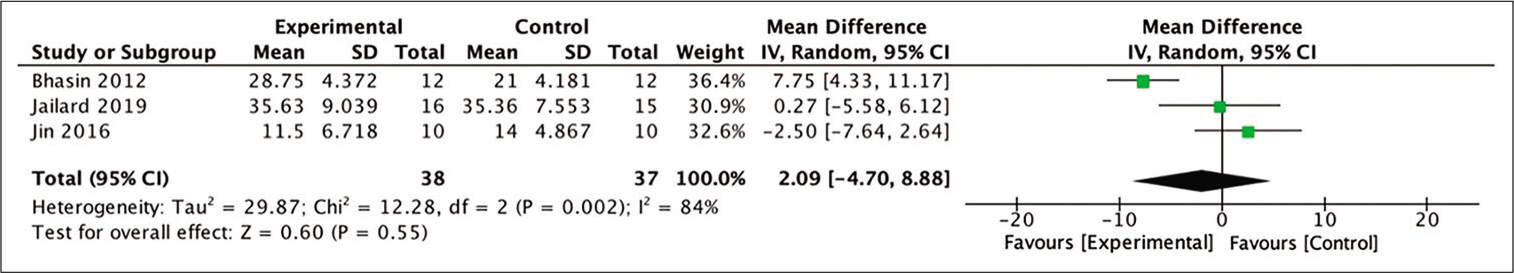
6-month BI
In terms of improvement of BI scores at 6-month posttherapy, three studies yielded extractable data for calculation out of the four studies included in the meta-analysis. The difference in mean BI scores in the stem cell therapy and control groups after 6 months was assessed for this analysis. The analysis indicated a more favorable outcome in those who received stem cell therapy, although these numbers were not statistically significant (MD = 2.09; 95% CI −4.70–8.88; P = 0.55; and I2 = 84%). [
Figure 6:
Calculation of mean difference in BI scores after 6 months between stem cell therapy and control groups in three studies. The mean difference was statistically greater in the stem cell therapy group, although this result was not statistically significant (MD = −0.27; 95% CI −0.52–0.17; P = 0.33; and I2 = 93%).
12-month outcome
12-month NIHSS
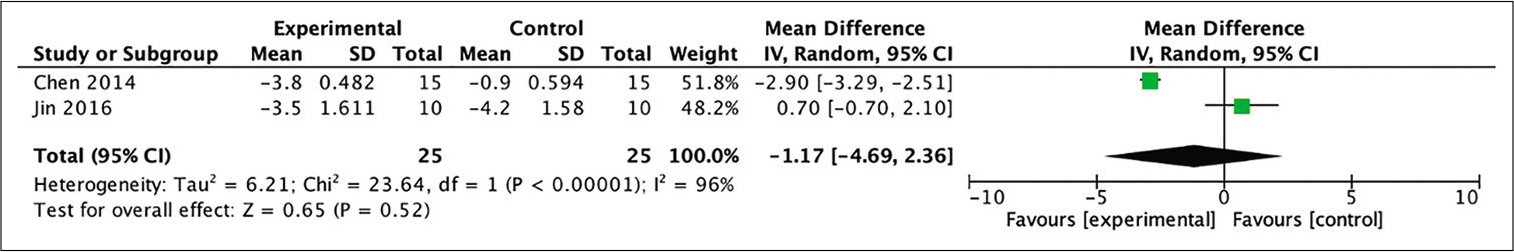
From four studies included in the meta-analysis, two studies were able to be analyzed for the analysis of improvement in the NIHSS score by calculating the mean difference between baseline and posttherapy for 12 months. The analysis of these two studies indicated a more favorable outcome (as indicated by the NIHSS scores at the 12th month) in the stem cell therapy compared to the control group (MD = −1.17; 95% CI −4.69– 2.36; P = 0.52; and I2 = 96%). However, these differences were not statistically significant, as shown in [
Figure 7:
Calculation of the mean difference in NIHSS scores after 12 months between the stem cell therapy and control groups in two studies. The mean difference was statistically greater in the stem cell therapy group, although this result was not statistically significant (MD = −1.17; 95% CI −4.69–2.36; P = 0.52; and I2 = 96%).
12-month mRS
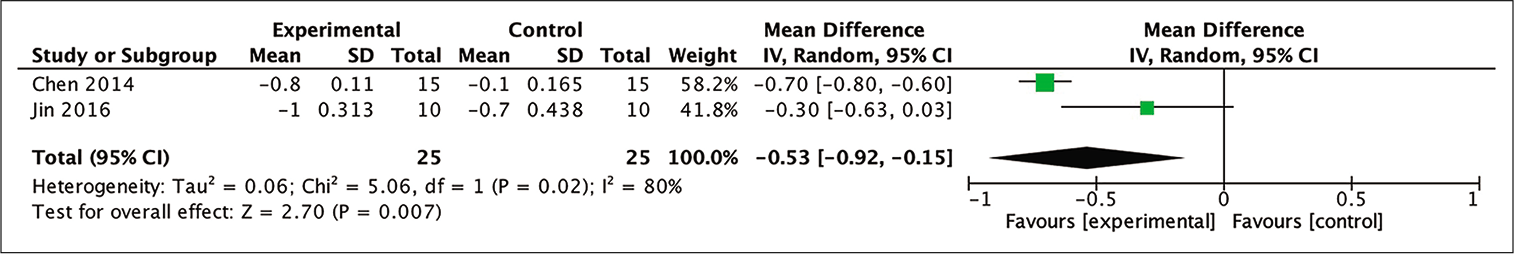
Two out of the four studies included in the meta-analysis were able to be analyzed to determine mRS score improvement by calculating the mean difference between the baseline and 12-month posttherapy mRS scores. From those two studies, a meta-analysis of the mean difference of mRS scores in the 12th month between the stem cell therapy and control groups indicated a statistically more favorable trend toward the group receiving stem cell administration (MD = −0.53; 95% CI −0.92–−0.15; P = 0.007; and I2 = 80%). This result is shown in [
Figure 8:
Calculation of the mean difference in mRS scores after 12 months between the stem cell therapy and control groups in two studies. The mean difference was statistically greater in the stem cell therapy group, and this result was statistically significant (MD = −0.53; 95% CI −0.92– −0.15; P = 0.007; and I2 = 80%).
24-month outcome
24-month NIHSS
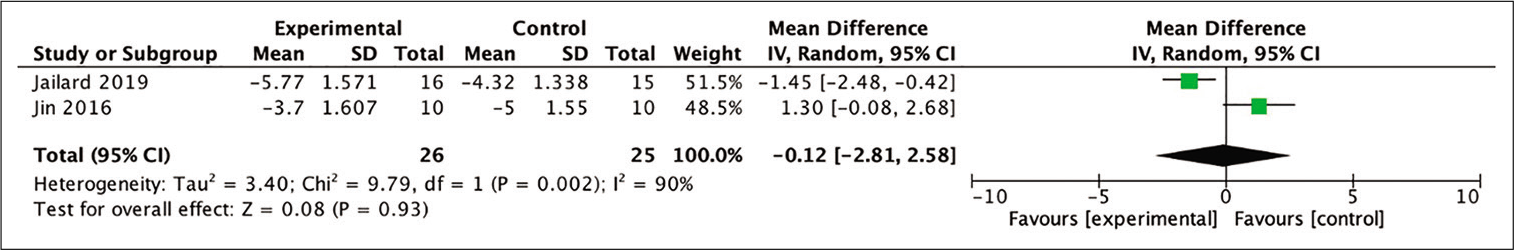
Assessment regarding the improvement of NIHSS score was analyzed from two out of the four studies included in the meta-analysis through the calculation of the mean difference between baseline and 24-month posttherapy scores. Each study yielded different conclusions and the pooled analysis indicated statistically insignificant results, with the result slightly in favor of the stem therapy in comparison with the control group (MD = −0.12; 95% CI −2.81–2.58; P = 0.93; and I2 = 90%). The forest-plot of the analysis is shown in [
Figure 9:
Calculation of the mean difference in NIHSS scores after 24 months between the stem cell therapy and control groups in two studies. The mean difference was statistically greater in the stem cell therapy group, although this result was not statistically significant (MD = −0.12; 95% CI −2.81–2.58; P = 0.93; and I2 = 90%).
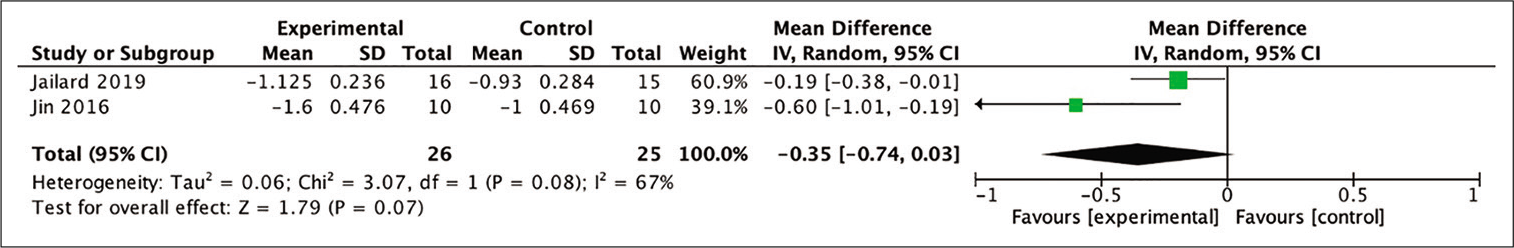
24-month mRS: The mean difference between the baseline and 24-month posttherapy mRS scores was able to be analyzed in two out of the four studies included in the meta-analysis. The obtained result of the analysis indicated a more favorable outcome in patients who received stem cell therapy group compared to those who did not (MD = −0.35; 95% CI −0.74–0.03; P = 0.07; and I2 = 67%). However, statistical analysis proved that this result had no statistical significance. The diagram of the analysis is shown in [
Figure 10:
Calculation of the mean difference in mRS scores after 24 months between the stem cell therapy and control groups in two studies. The mean difference was statistically greater in the stem cell therapy group, although this result was not statistically significant (MD = −0.35; 95% CI −0.74–0.03; P = 0.07; and I2 = 67%).
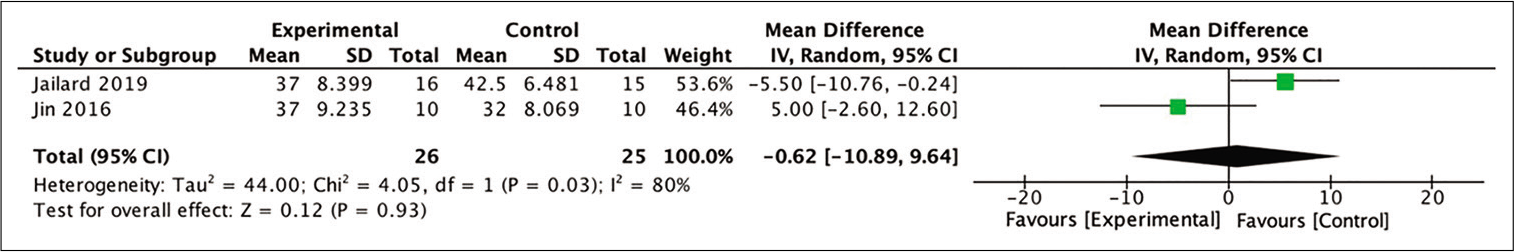
24-month BI
In all four studies included in the meta-analysis, two were able to be included in the meta-analysis for the improvement in BI scores by calculating the mean difference between baseline and 24-month posttherapy scores. The pooled analysis of those two studies yielded a statistically insignificant difference in outcomes after 24 months of observation between those groups. The result showed a slightly more favorable outcome for those who received stem cell therapy compared to the control, although it did not reach significance (MD = −0.62; 95% CI −10.89–9.64; P = 0.93; and I2 = 80%). The forest-plot of this analysis is shown in [
Figure 11:
Calculation of the mean difference in BI scores after 24 months between the stem cell therapy and control groups in two studies. The mean difference was statistically greater in the control group, although this result was not statistically significant (MD = −0.62; 95% CI −10.89–9.64; P = 0.93; and I2 = 80%).
DISCUSSION
Outcome improvement after administration of stem cell therapy
6-month outcome
Improvements in outcome were measured 6-, 12-, and 24-month posttherapy, by comparing the stem cell group to the control group. Analysis of the 6-month improvement of the NIHSS suggested favorable results for the stem cell group. The three studies included in the meta-analysis also showed favorable results for the stem cell group. Hence, stem cell therapy can show a significant improvement in neurological deficits after 6 months compared to control groups thus indicating that significant neural tissue repair occurs in the brain, which is the main target of stem cell therapy.[
In regard to the improvement of mRS analysis after 6 months, the trend also showed a favorable outcome toward the stem cell group, but this result was not statistically significant. A forest-plot analysis showed that the study by Jaillard et al. (2020) was the only study out of three studies that suggested more favorable results for the control group.[
Analysis of the improvement in BI score after 6 months showed more favorable results in the stem cell group, although this number was also not statistically significant. The study by Jin et al. (2017) showed favorable results for the control group, in contrast to the two other studies showing favorable results for the stem cell group.[
12-month outcome
The 12-month NIHSS outcome analysis showed favorable results for the stem cell group although these were not statistically significant. The results of this analysis need to be interpreted with caution; however, since only two studies conducted a meta-analysis for this 12-month NIHSS analysis.[
Similarly, analysis of the 12-month mRS scores showed a more statistically significant favorable outcome in the stem cell group compared to the control group. Both included studies showed these results.[
24-month outcome
The outcome analysis of 24-month NIHSS scores showed more favorable results in the stem cell group, but it was not statistically significant. The results of this analysis also need to be interpreted with caution since only two studies conducted a meta-analysis on this 24-month NIHSS analysis.[
A quantitative analysis of the improvement in mRS scores after 24 months revealed better results from the stem cell group compared to the control group, although this was not statistically significant. Both studies show a trend toward the stem cell group, but Jaillard et al. (2019) showed insignificant results. More disappointing results were found in the 24-month BI analysis, which showed a favorable trend toward the control group, although this was not statistically significant.[
The results of these two analyses support the findings from the study by Jaillard et al. described previously in which delayed MSC administration may affect the outcome. Despite this study showing evidence of the hypotheses of delayed continuous improvement in outcome of stem cell therapy, as we can see in mRS that favors stem cell group after 24 months, although it is still insignificant.[
Adverse events
Among the studies chosen for this review, a total of 467 adverse events were reported; 27.18% or 103 of these adverse events were serious. These statistics should be interpreted with caution; however, since not all studies reported adverse events as part of their research.[
CONCLUSION
According to our review, stem cell therapy for stroke cases showed a better outcome than standard conservative therapy alone, although our analysis shows that many factors can influence the outcome, and significant effects can only be seen after several months. The results of this study suggest that stem cell therapy has promising efficacy and is associated with a relatively low rate of serious adverse events. However, a larger and more targeted study comparing outcomes between stem cells therapy and conventional therapy is needed to strengthen our conclusions. Such a study needs to compare routes of stem cell administration, types of stem cell, timing from the onset of stroke to intervention, and stem cell dosage, as no studies have previously compared these factors directly.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018. 378: 708-18
2. Banerjee S, Bentley P, Hamady M, Marley S, Davis J, Shlebak A. Intra-arterial immunoselected CD34+ stem cells for acute ischemic stroke. Stem Cells Transl Med. 2014. 3: 1322-30
3. Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005. 57: 874-82
4. Bang OY. Clinical trials of adult stem cell therapy in patients with ischemic stroke. J Clin Neurol. 2016. 12: 14-20
5. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP. Heart disease and stroke statistics 2019 update: A report from the American heart association. Circulation. 2019. 139: e139-596
6. Bhasin A, Srivastava MV, Bhatia R, Mohanty S, Kumaran SS, Bose S. Autologous intravenous mononuclear stem cell therapy in chronic ischemic stroke. J Stem Cells Regen Med. 2012. 8: 181-9
7. Bhatia V, Gupta V, Khurana D, Sharma RR, Khandelwal N. Randomized assessment of the safety and efficacy of intra-arterial infusion of autologous stem cells in subacute ischemic stroke. AJNR Am J Neuroradiol. 2018. 39: 899-904
8. Boltze J, Ayata C. Challenges and controversies in translational stroke research an introduction. Transl Stroke Res. 2016. 7: 355-7
9. Chen DC, Lin SZ, Fan JR, Lin CH, Lee W, Lin CC. Intracerebral implantation of autologous peripheral blood stem cells in stroke patients: A randomized phase II study. Cell Transplant. 2014. 23: 1599-612
10. Chung J, Chang WH, Bang OY, Moon GJ, Kim SJ, Kim SK. Efficacy and safety of intravenous mesenchymal stem cells for ischemic stroke. Neurology. 2021. 96: e1012-23
11. Detante O, Jaillard A, Moisan A, Barbieux M, Favre IM, Garambois K. Biotherapies in stroke. Rev Neurol. 2014. 170: 779-98
12. Gautam J, Alaref A, Hassan A, Kandel RS, Mishra R, Jahan N. Safety and efficacy of stem cell therapy in patients with ischemic stroke. Cureus. 2020. 12: e9917
13. Hess DC, Wechsler LR, Clark WM, Savitz SI, Ford GA, Chiu D. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017. 16: 360-8
14. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M.editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). London, England: Cochrane; 2020. p.
15. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ.editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. London, England: The Cochrane Collaboration; 2011. p.
16. Honmou O, Houkin K, Matsunaga T, Niitsu Y, Ishiai S, Onodera R. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011. 134: 1790-807
17. Jaillard A, Hommel M, Moisan A, Zeffiro TA, Favre-Wiki IM, Barbieux-Guillot M. Autologous mesenchymal stem cells improve motor recovery in subacute ischemic stroke: A randomized clinical trial. Transl Stroke Res. 2020. 11: 910-23
18. Jiang Y, Zhu W, Zhu J, Wu L, Xu G, Liu X. Feasibility of delivering mesenchymal stem cells via catheter to the proximal end of the lesion artery in patients with stroke in the territory of the middle cerebral artery. Cell Transpl. 2013. 22: 2291-8
19. Jin Y, Ying L, Yu G, Nan G. Analysis of the long-term effect of bone marrow mononuclear cell transplantation for the treatment of cerebral infarction. Int J Clin Exp Med. 2017. 10: 3059-68
20. Kalladka D, Sinden J, Pollock K, Haig C, McLean J, Smith W. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): A phase 1, first-in-man study. Lancet. 2016. 388: 787-96
21. Kawabori M, Shichinohe H, Kuroda S, Houkin K. Clinical trials of stem cell therapy for cerebral ischemic stroke. Int J Mol Sci. 2020. 21: 7380
22. Laskowitz DT, Bennett ER, Durham RJ, Volpi JJ, Wiese JR, Frankel M. Allogeneic umbilical cord blood infusion for adults with ischemic stroke: Clinical outcomes from a phase 1 safety study. Stem Cells Transl Med. 2018. 7: 521-9
23. Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells (Dayton Ohio). 2010. 28: 1099-106
24. Lo CK, Mertz D, Loeb M. Newcastle-Ottawa scale: Comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014. 14: 45
25. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg. 2010. 8: 336-41
26. Morgenstern LB, Staub L, Chan W, Wein TH, Bartholomew LK, King M. Improving delivery of acute stroke therapy: The TLL temple foundation stroke project. Stroke. 2002. 33: 160-6
27. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018. 378: 11-21
28. Phipps MS, Cronin CA. Management of acute ischemic stroke. BMJ. 2020. 368: l6983
29. Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: A guideline for healthcare professionals from the American. Stroke. 2015. 46: 3020-35
30. Prasad K, Mohanty S, Bhatia R, Srixivastava MV, Garg A, Srivastava A. Autologous intravenous bone marrow mononuclear cell therapy for patients with subacute ischaemic stroke: A pilot study. Indian J Med Res. 2012. 136: 221-8
31. Prasad K, Sharma A, Garg A, Mohanty S, Bhatnagar S, Johri S. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: A multicentric, randomized trial. Stroke. 2014. 45: 3618-24
32. Qiao LY, Huang FJ, Zhao M, Xie JH, Shi J, Wang J. A two-year follow-up study of cotransplantation with neural stem/ progenitor cells and mesenchymal stromal cells in ischemic stroke patients. Cell Transpl. 2014. 23: 65-72
33. Savitz SI, Yavagal D, Rappard G, Likosky W, Rutledge N, Graffagnino C. A phase 2 randomized, sham-controlled trial of internal carotid artery infusion of autologous bone marrow-derived ALD-401 cells in patients with recent stable ischemic stroke (recover-stroke). Circulation. 2019. 139: 192-205
34. Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Kim AS, Johnson JN. Two-year safety and clinical outcomes in chronic ischemic stroke patients after implantation of modified bone marrow-derived mesenchymal stem cells (SB623): A phase 1/2a study. J Neurosurg. 2019. 131: 1462-72
35. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M.editors. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016. p. i4919
36. 37. Vahidy FS, Haque ME, Rahbar MH, Zhu H, Rowan P, Aisiku IP. Intravenous bone marrow mononuclear cells for acute ischemic stroke: Safety, feasibility, and effect size from a phase I clinical trial. Stem Cells. 2019. 37: 1481-91 38. Wardlaw JM, Murray V, Berge E, del Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2014. 2014: CD000213 39. Zhang G, Li Y, Reuss JL, Liu N, Wu C, Li J. Stable intracerebral transplantation of neural stem cells for the treatment of paralysis due to ischemic stroke. Stem Cells Transl Med. 2019. 8: 999-1007 40. Zheng H, Zhang B, Chhatbar PY, Dong Y, Alawieh A, Lowe F. Mesenchymal stem cell therapy in stroke: A systematic review of literature in pre-clinical and clinical research. Cell Transplantation. 2018. 27: 1723-30