Abstract
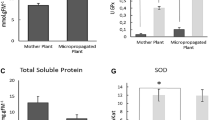
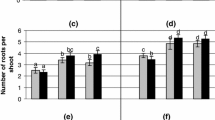
Present study describes rapid in vitro propagation of Caralluma tuberculata, a traditional medicinal plant, and antioxidant potential of calli and plants extracts. The highest callus induction rate (93.3%) with maximum weight of calli 5.2 g was achieved from shoot tip explants on MS medium supplemented with 9.04 μM 2,4-D and 4.44 μM BA. The maximum shoot induction rate (71.1%) with mean number of shoots 3.66 ± 1.53 and 4.6 cm average shoot length was observed on 13.32 μM BA, 4.52 μM 2,4-D and 2.89 μM GA3 appended in MS medium. The developed shoots were best rooted in the presence of 5.07 μM IAA with 3.0 ± 0.15 roots per plantlet. The plants were successfully acclimatized under in vivo conditions. The plants and calli extracts exhibited good antioxidant activities, however, plant extract activities were more pronounced. The phenolic compounds in plant and calli extracts were 0.16% and 0.057%, respectively. While the flavonoids were 0.092% in plant and 0.039% in calli extract. Total Phenolics, flavonoids; DPPH radical scavenging activity and reducing power potential distributed among different fractions depending upon polarity of the solvent. The highest DPPH scavenging activity and reducing power was exhibited by water fractions; 4.95 mg/mL and 0.729 OD at 10 mg/mL, respectively. The micropropagation protocol can be successfully used for large-scale multiplication and conservation of germplasm of this threatened plant. Furthermore, antioxidant value describes importance of this valuable plant as food and medicine.
Similar content being viewed by others
References
Abdel-Sattar E., Ahmed A.A., Hegazy F.M.E., Farag M.A. & Al-Yahya M.A. 2007. Acylated pregnane glycosides from Caralluma russeliana. Phytochem. 68: 1459–1463.
Al-Yahya M.A., Abdel-Sattar E. & Guittet E. 2000. Pregnane glycosides from Caralluma russeliana. J. Nat. Prod. 63: 1451–1453.
Anjum S., Zia M. & Chaudhary M.F. 2006. Investigations of different strategies for high frequency regeneration of Dendrobium malones ‘Victory’. African J. Biotechnol 5: 1738–1743.
Ansari N.M., Houlihan L., Hussain B. & Pieroni A. 2005. Antioxidant activity of five vegetables traditionally consumed by South-Asian migrants in Bradford, Yorkshire, UK. Phytother. Res. 19: 907–911.
Aruna V., Kiranmai C., Karuppusamy S. & Pullaiah T. 2009. Micropropagation of three varieties of Caralluma adscendens via nodal explants. J. Plant Biochem. Biotech. 18: 121–123.
Bader A., Braca A., DeTommasi N. & Morelli I. 2003. Further constituents from Caralluma negevensis. Phytochem. 62: 1277–1281.
Bais H.P., George J. & Ravishankar G.A. 2000. In vitro propagation of Decalepis hamiltonii Wight & Arn., an endangered shrub, through axillary bud cultures. Curr. Sci. 79: 408–410.
Bao J.S., Cai Y.Z., Sun M., Wang G.Y. & Corke H. 2005. Anthocyanins flavonols and free radical scavenging activity of Chinese bayberry Myrica rubra. extracts and their color properties and stability. J. Agric. Food Chem. 53: 2327–2332.
Bibi Y., Nisa S., Chaudhary F.M. & Zia M. 2011a. Antibacterial activity of some selected medicinal plants of Pakistan. BMC Comp. Alt. Med. 11: 52.
Bibi Y., Zia M., Nisa S., Habib D., Waheed A. & Chaudhary F.M. 2011b. Regeneration of Centella asiatica plants from non-embryogenic cell lines and evaluation of antibacterial and antifungal properties of regenerated calli and plants. J. Biol. Engin. 5: 13.
Bravo L. 1998. Polyphenols: chemistry, dietary sources, metabolism and nutritional significance. Nutrition Rev. 56: 317–333.
Chattopadhyay S., Datta S.K. & Ray M. 1992. In vitro effect of NH4 NO3 on growth and alkaloid content of Tylophora indica Merr. Phytomorphol. 42: 134–144
Chengalaryan K., Mhaske V.B. & Hazra S. 1997. High frequency conversion of abnormal peanut somatic embryos. Plant Cell Rep. 16: 783–786.
Dunbar K., Wilson K., Petersen B. & Biesboer D. 1986. Identification of laticifers in embryoids derived from callus and suspension of cultures of Asclepias species Asclepiadaceae. Am. J. Bot. 73: 847–851.
Gaj M.D. 2004. Factors influencing somatic embryogenesis induction and plant regeneration with particular reference to Arabidopsis thaliana L., Heynh. Plant Growth Reg. 43: 27–47.
Gazzarrini S. & McCourt P. 2003. Cross-talk in plant hormone signaling: what Arabidopsis mutants are telling us. Ann. Bot. 91: 605–612.
Grzegorczyk I., Matkowski A. & Wysokińska H. 2007. Antioxidant activity of extracts from in vitro cultures of Salvia officinalis L. Food Chem. 104: 536–541
Habib D., Chaudhary M.F. & Zia M. 2013. The study of ascorbate peroxidase, catalase and peroxidase during in vitro regeneration of Argyrolobium roseum. Appl. Biochem. Biotechnol. DOI: 10.1007/s12010-013-0591-6
Hu K., Yao X.S., Dong A.J., Kobayashi H., Iwasaki S. & Jing Y.K. 1999. New pregnane glycosides from Dioscorea collettii. J. Natural Prod. 62: 299–301.
Jiménez V.M. 2001. Regulation of in vitro somatic embryogenesis with emphasis on the role of endogenous hormones. Rev. Bras. Fisiol. Veg. 13: 196–223.
Kähkönen M.P., Hopia A.I., Vuorela H.J., Rauha J.P., Pihlaja K., Kujala T.S. & Heinonen M. 1999. Antioxidant activity of plant extracts containing phenolic compounds. J. Agri. Food Chem. 47: 3954–3962.
Kuda T., Tsunekawa M., Goto H. & Araki Y. 2005. Antioxidant properties of four edible algae harvested in the Noto Peninsula, Japan. J. Food Comp. Anal. 18: 625–633.
Lin L.J., Lin L.Z., Gil R.R., Cordell G.A., Ramesh M., Srilatha B., Reddy B. & Rao A.V.N.A. 1994. Pregnane glycosides from Caralluma umbellata. Phytochem. 35: 1549–1553.
Liu X., Zhao M., Wang J., Yang B. & Jiang Y. 2008. Antioxidant activity of methanolic extract of emblica fruit Phyllanthus emblica L.. from six regions in China. J. Food Comp. Anal. 21: 219–228.
Ma G., da Silva J.A.T. & Wu G. 2011. Direct adventitious shoot formation from apical shoot explants of Euphorbia tirucalli. J. Plant Growth Reg. 30: 114–116.
Mahender S.R. & Shekhawat N.S. 2013. In vitro regeneration in Sarcostemma acidum Roxb. — an important medicinal plant of semi-arid ecosystem of Rajasthan, India. Physiol. Mol. Biol. Plants 19: 269–275.
Martin K.P. 2002. Rapid propagation of Holostemma ada-kodien Schult., a rare medicinal plant, through axillary bud multiplication and indirect organogenesis. Plant Cell Rep. 21: 112–117.
Matsingou T.C., Petrakis N., Kapsokefalou M. & Salifoglou A. 2003. Antioxidant activity of organic extracts from aqueous infusion of sage. J. Agri. Food Chem. 51: 6696–6701.
Moreno M.I.N., Isla M.I., Sampietro A.R. & Vattuone M.A. 2000. Comparison of the free radical-scavenging activity of propolis from several regions of Argentina. J. Ethnopharm. 71: 109–114.
Murashige T. & Skoog F. 1962. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol. Plant 15: 473–497.
Naima B. & Taoufik K. 2006. Micropropagation of Teucrium stocksianum and Caralluma arabica — two endangered medicinal plants. Revue des Régions Arides — Numéro spécial — Actes du séminaire international les Plantes à Parfum, Aromatiques et Médicinales SIPAM.
Negi P.S., Jayaprakasha G.K. & Jena B.S. 2003. Antioxidant and antimutagenic activities of pomegranate peel extracts. Food Chem. 80: 393–397.
Nessler C.L. 1982. Somatic embryogenesis in the opium poppy, Papaver somniferum. Physiol. Plant. 55: 453–458.
Phulwaria M., Shekhawat N.S., Rathore J.S. & Singh P.R. 2013. An efficient in vitro regeneration and ex vitro rooting of Ceropegia bulbosa Roxb. — A threatened and pharmaceutical important plant of Indian Thar Desert. Indust. Crops Prod. 42: 25–29.
Piacente S., Belisario M.A., Del-Castillo H., Pizza C. & De Feo V. 1998. Croton ruizianus: platelet proaggregating activity of two new pregnane glycosides. J. Nat. Prod. 61: 318–322.
Pramanik T.K. & Datta S.K. 1986. Plant regeneration and ploidy variation in culture derived plants of Asclepias curassavica L. Plant Cell Rep. 5: 219–222.
Qiu S.X., Cordell G.A., Kumar B.R., Rao Y.N., Ramesh M. & Kokate C. 1999. Studies on the C-21 steroids from asclepiadaceous plants bisdesmosidic pregnane glycosides from Caralluma lasiantha. Phytochem. 50: 485–491.
Qiu S.X., Lin L.Z., Cordell G.A., Ramesh M., Kumar B.R., Radhakrishna M., Mohan G.K., Reddy B.M., Rao Y.N., Srinivas B., Thomas N.S. & Rao A.V.N.A. 1997. Acylated C-21 steroidal bisdesmosidic glycosides from Caraluma umbellate. Phytochem. 46: 333–340.
Radhakrishnan R., Zakaria M., Islam M.W., Liu X.M., Chan K., Habibullah M., Raghu R.D., SriRama M.K. & Pullaiah T. 2002. In vitro propagation of Cynanchum callialatum. J. Trop. Med. Plants 3: 233–238.
Rajendran R. & Ramaswamy K. 2004. Caralluma Extract Products and Processes for Making the Same. United States Patent and Trademark Office USPTO, U.S. Patent Documents 6376657.
Rashid H.S., Khan S.A., Zia M., Chaudhary M.F. & Hanif Z.C. 2009. Callus induction and rgeneration in elite sugarcane cultivar HSF-240. Pak. J. Bot. 41: 1645–1649.
Riaz U.R., Zia M. & Chaudhary M.F. 2007. Tissue culture studies of Caralluma tuberculata: an endangered plant. Plant Biology Meeting Abstract, 35048.
Roy A.T., Koutoulis A. & De D.N. 2000. Cell suspension culture and plant regeneration in the latex-producing plant, Calotropis gigantea Linn., R. Br. Plant Cell Tiss. Org. Cult. 63: 15–22.
Shimada K., Fujikawa K., Yahara K. & Nakamura T. 1992. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 40: 945–948.
Smetanska I. 2008. Production of secondary metabolites using plant cell cultures. Advances in Biochemical Engineering/ Biotechnology, vol. 111. Springer-Verlag, Berlin, pp. 187–228.
Sreelatha V.R., Rani S.S., Reddy P.V.K., Naveen M., Ugraiah A. & Pullaiah T. 2009. In vitro propagation of Caralluma sarkariae Lavranos & Frandsen — An endemic and endangered medicinal plant. Indian J. Biotechnol. 8: 236–239.
Sudha C.G., Krishnan P.N. & Pushpangadan P. 1998. In vitro propagation of Holostemma annulare Roxb., K. Schum, a rare medicinal plant. In vitro Cell Dev. Biol. Plant 33: 57–63.
Templeton J.F., Ling Y., Zeglam T.H. & LaBella F.S. 1993. Synthesis of 20-hydroxy-, 20-amino-, and 20 nitro-14-hydroxy-21-nor-5b, 14bpregnane C-3 glycosides and related derivatives: structure-activity relationships of pregnanes that bind to the Digitalis receptor. J. Med. Chem. 36: 42–45.
Ugraiah A., Karuppusamy S. & Pullaiah T. 2011. Micropropagation of Marsdenia brunoniana Wight & Arn. -A rare antidiabetic plant. Plant Tiss. Cult. Biotechnol. 21: 89–93.
Velioglu Y.S., Mazza G., Gao L. & Oomah B.D. 1998. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agri. Food Chem. 46: 4113–4117.
Vikrant A.R. 2002. Induction of multiple shoots by thidiazuron from caryopsis cultures of minor millet Paspalum scrobiculatum L., and its effect on the regeneration of embryogenic callus cultures. Plant Cell Rep. 21: 9–13.
Wang J. & Bao M.Z. 2007. Plant regeneration of pansy Viola wittrockiana, ‘Caidie’ via petiole-derived callus. Scientia Horticult. 111: 266–270.
Wilson H.M., Eisa M.Z. & Irwin S.W.B. 1976. The effects of agitated liquid medium on in vitro cultures of Hevea brasiliensis. Physiol. Plant. 36: 399–402.
Yen G.C. & Chen H.Y. 1995. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agri. Food Chem. 43: 27–32.
Zakaria M.N.M., Islam M.W. & Radhakrishnan R. 2002. Antigastric ulcer and cytoprotective properties of Caralluma arabica. Pharm. Biol. 40: 225–230.
Zhang X., daSilva J.A.T., Duan J., Deng R.F., Xu X.L. & Ma G.H. 2012. Endogenous hormone levels and anatomical characters of haustoria in Santalum album L. seedlings before and after attachment to the host. J. Plant Physiol. 169: 859–866.
Zia M., Mannan A. & Chaudhary M.F. 2007. Effect of amino acids and plant growth regulators on artenisinin production in the callus of Artemisia absinthium. Pak. J. Bot. 39: 799–805.
Zia M., Rizvi Z.F., Rehman R.U. & Chaudhary M.F. 2010. Micropropagation of two Pakistani soybean Glycine max L. cultivars from mature seeds cotyledon nodes. Spanish J. Agri. Res. 8: 448–453.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rehman, R.U., Chaudhary, M.F., Khawar, K.M. et al. In vitro propagation of Caralluma tuberculata and evaluation of antioxidant potential. Biologia 69, 341–349 (2014). https://doi.org/10.2478/s11756-013-0322-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-013-0322-z