Abstract
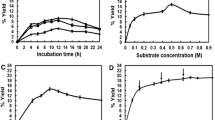
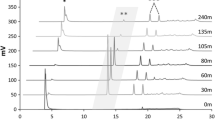
Two sucrose phosphorylases were employed for glycosylation of carboxylic acid compounds. Streptococcus mutans sucrose phosphorylase showed remarkable transglycosylating activity, especially under acidic conditions. Leuconostoc mesenteroides sucrose phosphorylase exhibited very weak transglycosylating activity. Three main products were detected from the reaction mixture using benzoic acid and sucrose as an acceptor and a donor molecule, respectively. These compounds were identified as 1-O-benzoyl α-d-glucopyranoside, 2-O-benzoyl α-d-glucopyranose, and 2-O-benzoyl β-d-glucopyranose by 1D-and 2D-NMR analyses of the isolated products and their acetylated products. Time-course analyses proved that 1-O-benzoyl α-d-glucopyranoside was initially produced by the transglycosylation reaction of the enzyme. 2-O-Benzoyl α-d-glucopyranose and 2-O-benzoyl β-d-glucopyranose were produced from 1-O-benzoyl α-d-glucopyranoside by intramolecular acyl migration reaction. S. mutans sucrose phosphorylase showed broad acceptor-specificity. This sucrose phosphorylase catalyzed transglycosylation to various carboxylic compounds such as short-chain fatty acids, hydroxy acids, dicarboxylic acids, and phenolic carboxylic acids. 1-O-Acetyl α-d-glucopyranoside was also enzymatically synthesized by transglucosylation reaction of the enzyme. The sensory test of acetic acid and the glucosides revealed that the sour taste of acetic acid glucosides was significantly lower than that of acetic acid.
Similar content being viewed by others
References
Clarke D.J. & Burchell B. 1994. The uridine diphosphate glucuronosyltransferase multigene family: function and regulation, pp. 3–43. In: Kauffman F.C. (ed.) Handbook of Experimental Pharmacology, Vol. 112, Conjugation-Deconjugation Reactions in Drug Metabolism and Toxicity, Springer-Verlag, Budapest.
Doudoroff M. 1943. Studies on the phosphorolysis of sucrose. J. Biol. Chem. 151: 351–361.
Fenselau C. 1994. Acyl glucuronides as chemically reactive intermediates, pp. 367–389. In: Kauffman F.C. (ed.) Handbook of Experimental Pharmacology, Vol. 112, Conjugation-Deconjugation Reactions in Drug Metabolism and Toxicity, Springer-Verlag, Budapest.
Fujii K., Iiboshi M., Yanase M., Takaha T. & Kuriki T. 2006. Enhancing the thermal stability of sucrose phosphorylase from Streptococcus mutans by random mutagenesis. J. Appl. Glycosci. 53: 91–97.
Funayama M., Arakawa H., Yamamoto R., Nishino T., Shin T. & Murao S. 1995. Effects of α-and β-arbutin on activity of tyrosinases from mushroom and mouse melanoma. Biosci. Biotechnol. Biochem. 59: 143–144.
Kishi M., Fukaya M., Tsukamoto Y., Nagasawa T., Takehana K. & Nishizawa N. 1999. Enhancing effect of dietary vinegar on the intestinal absorption of calcium in ovariectomized rats. Biosci. Biotechnol. Biochem. 63: 905–910.
Kitao S. & Sekine H. 1994. α-d-Glucosyl transfer to phenolic compounds by sucrose phosphorylase from Leuconostoc mesenteroides and production of α-arbutin. Biosci. Biotechnol. Biochem. 58: 38–42.
Kitaoka K., Takahashi H., Hara K., Hashimoto H., Sasaki T. & Taniguchi H. 1994. Purification and characterization of sucrose phosphorylase from Leuconostoc mesenteroides ATCC 12291 cells, and disaccharides synthesis by the enzyme. Oyo Toshitsu Kagaku 41: 165–172.
Kometani T., Terada Y., Nishimura T., Hiroshi T. & Okada S. 1994. Transglycosylation to hesperidin by cyclodextrin glucanotransferase from an alkalophilic Bacillus species in alkaline pH and properties of hesperidin glycosides. Biosci. Biotechnol. Biochem. 58: 1990–1994.
Kondo S., Tayama K., Tsukamoto Y., Ikeda K. & Yamori Y. 2001. Antihypertensive effects of acetic acid and vinegar on spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 65: 2690–2694.
Mieyal J.J. & Abeles R.H. 1972. Disaccharide Phosphorylases, pp. 515–532. In: Boyer P.D. (ed.), The Enzymes, Vol. 7, 3rd Ed., Academic Press, New York.
Mirza O., Skov L.K., Sprogøe D., van den Broek L.A., Beldman G., Kastrup J.S. & Gajhede M. 2006. Structural rearrangements of sucrose phosphorylase from Bifidobacterium adolescentis during sucrose conversion. J. Biol. Chem. 281: 35576–35584.
Nakano H., Kiso T., Okamoto K., Tomita T., Manan M.B. & Kitahata S. 2003. Synthesis of glycosyl glycerol by cyclodextrin glucanotransferases. J. Biosci. Bioeng. 95: 583–588.
Nishimura T., Kometani T., Takii H., Terada Y. & Okada S. 1994. Purification and some properties of α-amylase from Bacillus subtilis X-23 that glucosylates phenolic compounds such as hydroquinone. J. Ferment. Bioeng. 78: 31–36.
Nomura K., Sugimoto K., Nishiura H., Ohdan K., Nishimura T., Hayashi H. & Kuriki T. 2008. Glucosylation of acetic acid by sucrose phosphorylase. Biosci. Biotechnol. Biochem. 72: 82–87.
Spahn-Langguth H. & Benet L.Z. 1992. Acyl glucuronides revisited: is the glucuronidation process a toxification as well as a detoxification mechanism? Drug Metab. Rev. 24: 5–48.
Sugimoto K., Nishimura T., Nomura K., Sugimoto K. & Kuriki T. 2003. Syntheses of arbutin-α-glycosides and a comparison of their inhibitory effects with those of α-arbutin and arbutin on human tyrosinase. Chem. Pharm. Bull. 51: 798–801.
Sugimoto K., Nomura K., Nishimura T., Kiso T., Sugimoto K. & Kuriki T. 2005. Syntheses of α-arbutin-α-glycosides and their inhibitory effects on human tyrosinase. J. Biosci. Bioeng. 99: 272–276.
Sugimoto K., Nomura K., Nishiura H., Ohdan K., Nishimura T., Hayashi H. & Kuriki T. 2007. Novel transglucosylating reaction of sucrose phosphorylase to carboxylic compounds such as benzoic acid. J. Biosci. Bioeng. 104: 22–29.
Takenaka F. & Uchiyama H. 2000. Synthesis of α-d-glucosylglycerol by α-glucosidase and some of its characteristics. Biosci. Biotechnol. Biochem. 64: 1821–1826.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sugimoto, K., Nomura, K., Nishiura, H. et al. Sucrose phosphorylases catalyze transglycosylation reactions on carboxylic acid compounds. Biologia 63, 1015–1019 (2008). https://doi.org/10.2478/s11756-008-0161-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-008-0161-5