Abstract
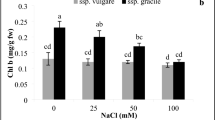
Salt stress affects growth and secondary metabolism in medicinal plants. There is no information about the impacts of salt stress on Dracocephalum kotschyi Boiss. Therefore, in this study, the 60-day old seedlings grown in coco peat and perlite were irrigated with various concentrations of NaCl (0, 25, 50, 75, and 100 mM) in accordance with a randomized complete block design for 3 weeks. The results showed that with increasing salt concentration, Na+ concentration was increased, but Ca2+ and K+ concentrations and fresh and dry weights were decreased in both roots and shoots. Besides, photosynthetic pigments and relative water content declined, whereas proline concentration and electrolyte leakage levels elevated in leaves under salinity stress. The activities of ascorbate peroxidase and guaiacol peroxidase enzymes were enhanced at lower salt concentrations but impaired under higher concentrations of NaCl. High-performance liquid chromatography showed that the contents of rosmarinic acid, luteolin, and apigenin were increased with aggravation of salt stress. The highest total phenolic and flavonoid contents and the rosmarinic acid, luteolin, and apigenin contents were verified at 75 mM NaCl that was concomitant with the most 2,2-diphenylpicrylhydrazyl scavenging activity in the leaves. In contrast, all these attributes decreased under 100 mM NaCl. In conclusion, the results unveiled that D. kotschyi is a moderate salt-tolerant plant and adaptive mechanisms adopted by this plant are including Na+ exclusion in roots, the activation of the antioxidant system, and the alteration of phenolic profile. This study also suggests that mild to moderate salinity can be employed as an elicitor to increasing some pharmaceutical important phenolic compounds in D. kotschyi.



Similar content being viewed by others
Abbreviations
- AF:
-
Apigenin flavone
- APX:
-
Ascorbate peroxidase
- Chl:
-
Chlorophyll
- EL:
-
Electrolyte leakage
- LF:
-
Luteolin flavone
- POD:
-
Guaiacol peroxidase
- RA:
-
Rosmarinic acid
- RDW:
-
Root dry weight
- RFW:
-
Root fresh weight
- R/S:
-
Root to shoot ratio
- RWC:
-
Relative water content
- SDW:
-
Shoot dry weight
- SFW:
-
Shoot fresh weight
- TFC:
-
Total flavonoid content
- TPC:
-
Total phenolic content
References
Abdallah SB, Aung B, Amyot L, Lalin I, Lachâal M, Karray-Bouraoui N, Hannoufa A (2016) Salt stress (NaCl) affects plant growth and branch pathways of carotenoid and flavonoid biosyntheses in Solanum nigrum. Acta Physiol Plant 38:72
Al-Ghamdi AA, Elansary HO (2018) Synergetic effects of 5-aminolevulinic acid and Ascophyllum nodosum seaweed extracts on Asparagus phenolics and stress related genes under saline irrigation. Plant Physiol Biochem 129:273–284
Amooaghaie R (2011) The effect of hydro and osmopriming on alfalfa seed germination and antioxidant defenses under salt stress. Afri J Biotechnol 10:6269–6275
Amooaghaie R, Tabatabaie F (2017) Osmopriming-induced salt tolerance during seed germination of alfalfa most likely mediates through H2O2 signaling and upregulation of heme oxygenase. Protoplasma 254:1791–1803
Bates LS, Waldren RP, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bazrafshan A, Ehsanzadeh P (2016) Evidence for differential lipid peroxidation and antioxidant enzyme activities in Sesamum indicum L. genotypes under NaCl salinity. J Agri Sci Tech 18:207–222
Bistgani ZE, Hashemi M, DaCosta M, Craker L, Maggi F, Morshedloo MR (2019) Effect of salinity stress on the physiological characteristics, phenolic compounds and antioxidant activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind Crop Prod 135:311–320
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Caretto S, Linsalata V, Colella G, Mita G, Lattanzio V (2015) Carbon fluxes between primary metabolism and phenolic pathway in plant tissues under stress. Int J Mol Sci 16:26378–26394
Chrysargyris A, Michailidi E, Tzortzakis N (2018) Physiological and biochemical responses of Lavandula angustifolia to salinity under mineral foliar application. Fron Plant Sci 9:489
Chrysargyris A, Solomou M, Petropoulos SA, Tzortzakis N (2019) Physiological and biochemical attributes of Mentha spicata when subjected to saline conditions and cation foliar application. J Plant Physiol 232:27–38
Çoban Ö, Baydar NG (2016) Brassinosteroid effects on some physical and biochemical properties and secondary metabolite accumulation in peppermint (Mentha piperita L.) under salt stress. Ind Crop Prod 86:251–258
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Eskandari H, Al-Mansour N, Ehsanpour AA (2018) Rosmarinic acid ameliorates the negative effects of salinity in in vitro-regenerated potato explants (Solanum tuberosum L.). Acta Physiol Plant 40:74
Estaji A, Roosta HR, Rezaei SA, Hosseini SS, Niknam F (2018) Morphological, physiological and phytochemical response of different Satureja hortensis L. accessions to salinity in a greenhouse experiment. J Appl Res Med Aromat Plants 10:25–33
Fathi M, Rezaei M (2013) Soil salinity in the central arid region of Iran: Esfahan Province. In: Developments in Soil Salinity Assessment and Reclamation, pp 141–153
Fattahi M, Bonfill M, Fattahi B, Torras-Claveria L, Sefidkon F, Cusido RM, Palazon J (2016) Secondary metabolites profiling of Dracocephalum kotschyi Boiss at three phenological stages using uni-and multivariate methods. J Appl Res Med Aromat Plants 3:177–185
Fattahi M, Nazeri V, Torras-Claveria L, Sefidkon F, Cusido RM, Zamani Z, Palazon J (2013) Identification and quantification of leaf surface flavonoids in wild-growing populations of Dracocephalum kotschyi by LC–DAD–ESI-MS. Food Chem 141:139–146
Gengmao Z, Quanmei S, Yu H, Shihui L, Changhai W (2014) The physiological and biochemical responses of a medicinal plant (Salvia miltiorrhiza L.) to stress caused by various concentrations of NaCl. PloS one 9:e89624
Gengmao Z, Yu H, Xing S, Shihui L, Quanmei S, Changhai W (2015) Salinity stress increases secondary metabolites and enzyme activity in safflower. Ind Crop Prod 64:175–181
Ghanadian M, Shamaeezadeh N, Mohammadi P, Mohajeri M (2018) A new validated high-performance liquid chromatography method for standardization of rosmarinic acid in Salvia extracts. J Herbmed Pharmacol 7:44–50
Ghorbannejad H, Amooaghaie R (2017) Differential changes of proline content and activities of antioxidant enzymes results in varied salt-tolerance in canola genotypes. J Genet Resour 3:36–46
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments: a review. Plant Signal Behav 7:1456–1466
Heydari P, Yavari M, Adibi P, Asghari G, Ghanadian S-M, Dida GO, Khamesipour F (2019) Medicinal properties and active constituents of Dracocephalum kotschyi and its significance in Iran: a systematic review. Evid Based Complement Alternat Med 2019:1–14
Isayenkov SV, Maathuis FJ (2019) Plant salinity stress: many unanswered questions remain. Front Plant Sci 10:80
Jahantigh O, Najafi F, Badi HN, Khavari-Nejad RA, Sanjarian F (2016) Changes in antioxidant enzymes activities and proline, total phenol and anthocyanine contents in Hyssopus officinalis L. plants under salt stress. Acta Biol Hung 67:195–204
Johnson H Jr (1980) Hydroponics: a guide to soilless culture, leaflet 2947. Division of agriculture and natural resources. Univ of California, Berkeley
Khodabakhsh F, Amooaghaie R, Mostajeran A (2014) Effect of hydro-and osmo-priming on membrane deterioration, proline accumulation and H2O2 scavenging enzymes in two salt stressed chickpea cultivars. Environ Eng Manag J (EEMJ) 13:619–626
Kleinwächter M, Selmar D (2015) New insights explain that drought stress enhances the quality of spice and medicinal plants: potential applications. Agron Sustain Dev 35:121–131
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Mahouachi J (2018) Long-term salt stress influence on vegetative growth and foliar nutrient changes in mango (Mangifera indica L.) seedlings. Sci Hortic 234:95–100
Maikai V, Kobo P, Maikai B (2010) Antioxidant properties of Ximenia americana. Afr J Biotechnol 9:7744–7746
Mekawy AMM, Abdelaziz MN, Ueda A (2018) Apigenin pretreatment enhances growth and salinity tolerance of rice seedlings. Plant Physiol Biochem 130:94–104
Menezes RV, Azevedo Neto A, Gheyi HR, Cova AM, Silva HH (2017) Tolerance of basil genotypes to salinity. J Agric Sci 9:283–295
Naderi S, Seraji M, Fakheri B (2015) Effects of salinity stress on proline, phenolic compounds and activity of antioxidant enzymes in Dracocephalum moldavica L. Elix Agric 82:32648–32651
Pękal A, Pyrzynska K (2014) Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal Methods 7:1776–1782
Ouzounidou G, Ilias I, Giannakoula A, Theoharidou I (2014) Effect of water stress and NaCl triggered changes on yield, physiology, biochemistry of broad bean (Vicia faba) plants and on quality of harvested pods. Biologia 69:1010–1017
Ouzounidou G, Skiada V, Papadopoulou KK, Stamatis N, Kavvadias V, Eleftheriadis E, Gaitis F (2015) Effects of soil pH and arbuscular mycorrhiza (AM) inoculation on growth and chemical composition of chia (Salvia hispanica L.) leaves. Braz J Bot 38:487–495
Rouphael Y, Kyriacou M, Carillo P, Pizzolongo F, Romano R, Sifola M (2019) Chemical eustress elicits tailored responses and enhances the functional quality of novel food Perilla frutescens. Molecules 24:185
Scagel CF, Lee J, Mitchell JN (2019) Salinity from NaCl changes the nutrient and polyphenolic composition of basil leaves. Ind Crop Prod 127:119–128
Selmar D, Kleinwächter M (2013) Influencing the product quality by deliberately applying drought stress during the cultivation of medicinal plants. Ind Crop Prod 42:558–566
Shafeiee M, Ehsanzadeh P (2019) Physiological and biochemical mechanisms of salinity tolerance in several fennel genotypes: existence of clearly-expressed genotypic variations. Ind Crop Prod 132:311–318
Singleton Vernon L, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol 299:152–178
Smart RE, Bingham GE (1974) Rapid estimates of relative water content. Plant Physiol 53:258–260
Taârit MB, Msaada K, Hosni K, Marzouk B (2012) Physiological changes, phenolic content and antioxidant activity of Salvia officinalis L. grown under saline conditions. J Sci Food Agric 92:1614–1619
Tabatabaei S, Ehsanzadeh P (2016) Photosynthetic pigments, ionic and antioxidative behaviour of hulled tetraploid wheat in response to NaCl. Photosynthetica 54:340–350
Tanaka H, Yamada S, Masunaga T, Yamamoto S, Tsuji W, Murillo-Amador B (2018) Comparison of nutrient uptake and antioxidative response among four Labiatae herb species under salt stress condition. Soil Sci Plant Nutr 64:589–597
Tounekti T, Hernández I, Müller M, Khemira H, Munné-Bosch S (2011) Kinetin applications alleviate salt stress and improve the antioxidant composition of leaf extracts in Salvia officinalis. Plant Physiol Biochem 49:1165–1176
Trivellini A, Lucchesini M, Maggini R, Mosadegh H, Villamarin TSS, Vernieri P, Mensuali-Sodi A, Pardossi A (2016) Lamiaceae phenols as multifaceted compounds: bioactivity, industrial prospects and role of “positive-stress”. Ind Crop Prod 83:241–254
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66
Yang L, Wen K-S, Ruan X, Zhao Y-X, Wei F, Wang Q (2018) Response of plant secondary metabolites to environmental factors. Molecules 23:762
Yang Y, Guo Y (2018) Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol 217:523–539
Zhou Y, Tang N, Huang L, Zhao Y, Tang X, Wang K (2018) Effects of salt stress on plant growth, antioxidant capacity, glandular trichome density, and volatile exudates of Schizonepeta tenuifolia Briq. Int J Mol Sci 19:252
Acknowledgments
This study was supported by the Plant Science Department of Shahrekord University, Iran. We thank Dr. Mehran Torkian for his advice in statistical analysis.
Author information
Authors and Affiliations
Contributions
This research has been completed with the collaboration of all authors. Farinaz Vafadar conducted the experiments, analyzed, and wrote the manuscript. Rayhaneh Amooaghaie designed and supervised this study and contributed to the writing and interpreting of the results of this manuscript. Parviz Ehsanzadeh was the co-supervisor, provided laboratory facilities for this research and edited manuscript. Mostafa Ghanadian advised measuring phenolic compounds, in particular by HPLC.
Corresponding author
Ethics declarations
Conflict of interest
The authors state that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vafadar, F., Amooaghaie, R., Ehsanzadeh, P. et al. Salinity stress alters ion homeostasis, antioxidant activities and the production of rosmarinic acid, luteolin and apigenin in Dracocephalum kotschyi Boiss. Biologia 75, 2147–2158 (2020). https://doi.org/10.2478/s11756-020-00562-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-020-00562-3