Abstract
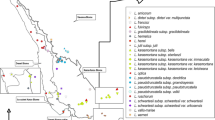
Few studies of genetic variation have been conducted on plants of the Pacific coastal desert and neighboring Andes of Peru, although the region has many endemic taxa. Enzyme electrophoresis was employed to examine allozyme diversities of four species of the family Malesher-biaceae, an endemic to the arid Andes and coastal desert. One population ofMalesherbia splendens and two ofM. tubulosa, both endemics of the department of Lima, one population ofM. weberbaueri var.weberbaueri, an endemic of the Andean department Huancavélica, and the two Lima populations ofM. scarlatiflora were studied. Fifteen loci were examined for all populations and an additional seven loci were resolved forM. weberbaueri andM. splendens. Malesherbia splendens, which is known from three populations, has a low mean number of alleles per locus (A), proportion of polymorphic loci (P), and expected heterozygosity (Hs) (A=1.214, P=0.214, Hs=0.057).Malesherbia weberbaueri (A=1.231, P=0.154, Hs=0.079) andM. scarlatiflora (A=1.364, P=0.273, Ht=0.083) both have average expected heterozygosities and relatively low mean numbers of alleles per locus and proportions of polymorphic loci. InM. tubulosa, all measures of genetic diversity are high in comparison with other endemics (A=1.818, P=0.364, Ht=0.206).Malesherbia tubulosa has high interpopulation differentiation, whereasM. scarlatiflora has low among-population diversity. The relatively low allozyme diversities, restricted habitats, narrow ranges ofM. splendens andM. weberbaueri, and the morphological similarities of all four species suggest that they evolved recently by founder events. Greater allozyme diversities inM. tubulosa could be attributable to its maintenance of larger populations in a greater variety of habitats.
Similar content being viewed by others
Literature Cited
Arft, A. M. &T. A. Ranker. 1998. Allopolyploid origin and population genetics of the rare orchidSpiranthes diluvialis. Amer. J. Bot. 85: 110–122.
Bretó, M. P., M. J. Asins &E. A. Carbonell. 1993. Genetic variability inLycopersicon species and their genetic relationships. Theor. Appl. Genet. 86: 113–120.
Crawford, D. J., E. Landolt, D. H. Les &E. Tepe. 1997. Allozyme variation and the taxonomy ofWolffiella. Aquatic Bot. 58: 43–54.
—,A. Segástegui A., T. F. Stuessy &I. Sánchez V. 1993. Variación aloenzimática en la rara especie endémica peruanaChuquiraga oblongifolia (Asteraceae). Arnaldoa 1: 73–76.
—,T. F. Stuessy, D. W. Haines, M. B. Cosner, M. Silva O. &P. Lopez. 1992. Allozyme diversity within and divergence among four species ofRobinsonia (Asteraceae: Senecioneae), a genus endemic to the Juan Fernandez Islands, Chile. Amer. J. Bot. 79: 962–966.
Davis, B. J. 1964. Dis-electrophoresis II. Methods and application to human serum proteins. Ann. New York Acad. Sci. 21: 404–427.
Godt, M. J. W. 1997. Genetic diversity in the endangered lilyHarperocallis flava and a close relative,Tofieldia racemosa. Conserv. Biol. 11: 361–366.
—,J. Walker &J. L. Hamrick. 1995. Genetic diversity in a threatened wetland species,Helonias bullata (Liliaceae). Conserv. Biol. 9: 596–604.
—,B. R. Johnson &J. L. Hamrick. 1996. Genetic diversity and population size in four rare southern Appalachian plant species. Conserv. Biol. 10: 796–805.
Gottlieb, L. D. 1973. Genetic differentiation, sympatric speciation, and the origin of a diploid species ofStephanomeria. Amer. J. Bot. 60: 545–553.
—. 1981. Electrophoretic evidence and plant populations. Progr. Phytochem. 7: 1–46.
Hamrick, J. L. &M. J. W. Godt. 1990. Allozyme diversity in plant species. Pages 43–63.In: A. H. D. Brown, M. T. Clegg, A. L. Kahler & B. S. Weir, itors. Plant population genetics, breeding, and genetic resources. Sinauer Associates, Sunderland, Massachusetts.
—&—. 1997. Effects of life history traits on genetic diversity in plant species. Pages 103–118.In: J. Silvertown, M. Franco & J. L. Harper, editors. Plant life histories: ecology, phylogeny, and evolution. Cambridge University Press, Cambridge.
Leberg, P. L. 1992. Effects of population bottlenecks on genetic diversity as measured by allozyme electrophoresis. Evolution 46: 477–494.
Lewis, P. O. &R. Whitkus. 1989. GeneStat (version 3.3) for microcomputers. Amer. Soc. Pl. Tax. Newslett. 2: 15–16.
Nei, M. 1973. Analysis of gene diversity in subdivided populations. Proc. Natl. Acad. Sci. U.S.A. 70: 3321–3323.
—,T. Maruyama &R. Chakraborty. 1975. The bottleneck effect and genetic variability in populations. Evolution 29: 1–10.
Percy, D. M. &Q. C. B. Cronk. 1997. Conservation in relation to mating system inNesohedyotis arborea (Rubiaceae), a rare endemic tree from St. Helena. Biol. Conserv. 80: 135–145.
Purdy, B. G., R. J. Bayer &S. E. MacDonald. 1994. Genetic variation, breeding system evolution, and conservation of the narrow sand dune endemicStellaria arenicola and the widespreadS. longipes (Caryophyllaceae). Amer. J. Bot. 81: 904–911.
Qiu, Y.-L. &C. R. Parks. 1994. Disparity of allozyme variation levels in threeMagnolia (Magnoliaceae) species from the southeastern United States. Amer. J. Bot. 81: 1300–1308.
Quiros, C. F., S. B. Brush, D. S. Douches, K. S. Zimmerer &G. Huestis. 1990. Biochemical and folk assessment of variability of Andean cultivated potatoes. Econ. Bot. 44: 254–266.
Rauh, W. 1985. The Peruvian—Chilean deserts. Pages 239–267.In: M. Evenari, I. Noy-Meir & D. W. Goodall, editors. Hot deserts and arid shrublands. Elsevier, Amsterdam.
Ricardi, M. 1967. Revision taxonómica de las Malesherbiáceas. Gayana, Bot. no. 16: 3–139.
Richards, C. &P. L. Leberg. 1996. Temporal changes in allele frequencies and a population's history of severe bottlenecks. Conserv. Biol. 10: 823–839.
Rick, C. M. &J. F. Fobes. 1975. Allozyme variation in the cultivated tomato and closely related species. Bull. Torrey Bot. Club 102: 375–384.
—,E. Kesicki, J. F. Fobes &M. Holle. 1976. Genetic and biosystematic studies on two new sibling species ofLycopersicon from interandean Perú. Theor. Appl. Genet. 47: 55–68.
Rieseberg, L. H., S. Zona, L. Aberbom &T. D. Martin. 1989. Hybridization in the island endemic, Catalina mahogany. Conserv. Biol. 3: 1–7.
Sahley, C. T. 1996. Bat and hummingbird pollination of an autotetraploid columnar cactus,Weberbauer-ocereus weberbaueri (Cactaceae). Amer. J. Bot. 83: 1329–1336.
Schwaegerle, K. E. &B. A. Schaal. 1979. Genetic variation and founder effect in the pitcher plantSarracenia purpurea L. Evolution 33: 1210–1218.
Singh, S. P., R. Nodari &P. Gepts. 1991. Genetic diversity in cultivated common bean: I. Allozymes. Crop Sci. 31: 19–23.
Tohme, J., D. Orlando G., S. Beebe &M. C. Duque. 1996. AFLP analysis of gene pools of a wild bean core collection. Crop Sci. 36: 1375–1384.
Weeden, N. F. &J. F. Wendel. 1989. Genetics of plant isozymes. Pages 46–72.In: D. E. Soltis & P. S. Soltis, editors. Isozymes in plant biology. Dioscorides Press, Portland, Oregon.
Wendel, J. F. &N. F. Weeden. 1989. Visualization and interpretation of plant isozymes. Pages 5–45.In: D. E. Soltis & P. S. Soltis, editors. Isozymes in plant biology. Dioscorides Press, Portland, Oregon.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gengler, K.M., Crawford, D.J. Genetic diversities of four little-known species of Malesherbia (Malesherbiaceae) endemic to the arid inter-Andean valleys of Peru. Brittonia 52, 303–310 (2000). https://doi.org/10.2307/2666581
Issue Date:
DOI: https://doi.org/10.2307/2666581