Abstract
Introduction The ADNI (Alzheimer’s Disease Neuroimaging Initiative) is a large longitudinal study of patients with probable Alzheimer’s disease (AD), patients with mild cognitive impairment (MCI) and healthy elderly controls followed for at least 2–3 years. Many participants in the ADNI are being treated with medications, and these may have beneficial or deleterious effects.
Objective The goal of the study was to characterize baseline medication use in the ADNI.
Methods Diagnosis, demographics, medication status, psychometric data and MRI measures of hippocampal volume and entorhinal cortex thickness were obtained for 818 participants from the ADNI cohort. Total number of medications, Beers list (potentially dangerous) medications and AD treatments were also tabulated. ANOVA and logistic regression were used to assess associations between baseline pharmacotherapy and diagnosis, demographics, and selected clinical and MRI variables.
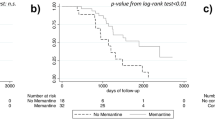
Results Of the 818 enrolled ADNI participants, 809 were available for analysis in the present study, including 184 patients with AD, 399 patients with MCI and 226 healthy elderly controls. Significant gender differences in recruitment were observed in the MCI group. The average number of medications per participant was 8 (SD 4) and 22% reported treatment with one or more Beers list medications. For symptomatic treatment of MCI or AD, donepezil and memantine were the most commonly reported drugs. As expected, MCI and AD patients with more severe impairment were more likely to be treated. Men received treatment more frequently than women. Older subjects and those with less education were less likely to receive treatment.
Conclusions AD and MCI participants from the ADNI cohort were being treated with polypharmacy and many were also taking one or more medications with the potential for adverse effects. Off-label use of cholinesterase inhibitors and/or memantine for MCI was common, with more severely affected patients most likely to receive treatment. Differences in the frequency of symptomatic treatment were also observed as a function of age, years of education, gender and disease severity.




Similar content being viewed by others
References
Alarcon T, Barcena A, Gonzalez-Montalvo JI, et al. Factors predictive of outcome on admission to an acute geriatric ward. Age Ageing 1999; 28(5): 429–32
Flaherty JH, Perry 3rd HM, Lynchard GS, et al. Polypharmacy and hospitalization among older home care patients. J Gerontol A Biol Sci Med Sci 2000; 55(10): M554–9
Satish S, Winograd CH, Chavez C, et al. Geriatric targeting criteria as predictors of survival and health care utilization. J Am Geriatr Soc 1996; 44(8): 914–21
Shorr RI, Ray WA, Daugherty JR, et al. Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas. Arch Intern Med 1997; 157(15): 681–6
Jensen GL, Friedmann JM, Coleman CD, et al. Screening for hospitalization and nutritional risks among community-dwelling older persons. Am J Clin Nutr 2001; 74(2): 201–5
Veehof LJ, Stewart RE, Meyboom-de Jong B, et al. Adverse drug reactions and polypharmacy in the elderly in general practice. Eur J Clin Pharmacol 1999; 55(7): 533–6
Cohen I, Rogers P, Burke V, et al. Predictors of medication use, compliance and symptoms of hypotension in a community-based sample of elderly men and women. J Clin Pharm Ther 1998; 23(6): 423–32
Goldberg RM, Mabee J, Chan L, et al. Drug-drug and drug-disease interactions in the ED: analysis of a high-risk population. Am J Emerg Med 1996; 14(5): 447–50
Nolan L, O’Malley K. Prescribing for the elderly: part I. Sensitivity of the elderly to adverse drug reactions. J Am Geriatr Soc 1988; 36(2): 142–9
Fick DM, Cooper JW, Wade WE, et al. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. [published erratum appears in Arch Intern Med 2004; 164 (3): 298]. Arch Intern Med 2003; 163(22): 2716–24
Doody RS, Geldmacher DS, Gordon B, et al., Donepezil Study G. Open-label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer disease. Arch Neurol 2001; 58(3): 427–33
Razadyne [package insert]. Titusville (NJ): Ortho-McNeil Neurologics, Inc. 2005
Exelon [package insert]. East Hanover (NJ): Novartis Pharmaceuticals, 2001
Winblad B, Grossberg G, Frolich L, et al. IDEAL: a 6-month, double-blind, placebo-controlled study of the first skin patch for Alzheimer disease. Neurology 2007; 69(4 Suppl. 1): S14–22
Cacabelos R, Llovo R, Fraile C, et al. Pharmacogenetic aspects of therapy with cholinesterase inhibitors: the role of CYP2D6 in Alzheimer’s disease pharmacogenetics. Curr Alzheimer Res 2007; 4(4): 479–500
Reisberg B, Doody R, Stoffler A, et al. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 2003; 348(14): 1333–41
Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA 2004; 291(3): 317–24
Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 2007 Aug; 6(8): 734–46
Raschetti R, Albanese E, Vanacore N, et al. Cholinesterase inhibitors in mild cognitive impairment: a systematic review of randomised trials. PLoS Med 2007; 4(11): e338
US Food and Drug Administration. Alert for healthcare professionals on galantamine hydrochloride (marketed as Reminyl). 2005 [online]. Available from URL: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm085186.htm [Accessed 2009 Jun 30]
Mueller SG, Weiner MW, Thal LJ, et al. The Alzheimer’s Disease Neuroimaging Initiative. Neuroimaging Clin N Am 2005; 15(4): 869–77
Mueller SG, Weiner MW, Thal LJ, et al. Ways toward an early diagnosis in Alzheimer’s disease: the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Alzheimers Dement 2005; 1(1): 55–66
Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage 1999; 9(2): 179–94
Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 2000; 97(20): 11050–5
Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33(3): 341–55
Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis: II. Inflation, flattening, and a surface-based coordinate system. Neuroimage 1999; 9(2): 195–207
Jack Jr CR, Bernstein MA, Fox NC, et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging 2008; 27(4): 685–91
Schwartz JB. The current state of knowledge on age, sex, and their interactions on clinical pharmacology. Clin Pharmacol Ther 2007; 82(1): 87–96
Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med 2006; 355(9): 873–84
ADAPT Group. Cardiovascular and cerebrovascular events in the randomized, controlled Alzheimer’s Disease Anti-Inflammatory Prevention Trial (ADAPT). PLOS Clin Trials 2006; 1(7): E33
Hulley SB, Grady D. The WHI estrogen-alone trial: do things look any better? JAMA 2004; 291(14): 1769–71
Preskorn SM. Clinical pharmacology of SSRIs. 1st ed. Wichita (KS): Professional Communications, 1996
Kay GG, Abou-Donia MB, Messer Jr WS, et al. Anti-muscarinic drugs for overactive bladder and their potential effects on cognitive function in older patients. J Am Geriatr Soc 2005; 53(12): 2195–201
Paterniti S, Dufouil C, Alperovitch A. Long-term benzo-diazepine use and cognitive decline in the elderly: the Epidemiology of Vascular Aging study. J Clin Psycho-pharmacol 2002; 22(3): 285–93
Kilborn MJ, Rathore SS, Gersh BJ, et al. Amiodarone and mortality among elderly patients with acute myocardial infarction with atrial fibrillation. Am Heart J 2002; 144(6): 1095–101
Potentially harmful drugs in the elderly: Beers’ list and more [letter]. Prescr Lett 2007; 23(9): 230907
Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI) clinical characterization. Neurology 2010; 74(3): 201–9
American Academy of Neurology. Dementia. Continuum 2007; 13(2): 13–58
Risacher S, Saykin A, West J, et al. Baseline MRI predictors for conversion from MCI to probable AD in the ADNI cohort. Curr Alzheimer Res 2009; 6(4): 347–61
Hirschman KB, Joyce CM, James BD, et al. Would caregivers of Alzheimer disease patients involve their relative in a decision to use an AD-slowing medication? Am J Geriatr Psychiatry 2005; 13(11): 1014–21
Karlawish JH, Casarett D, Klocinski J, et al. How do AD patients and their caregivers decide whether to enroll in a clinical trial? Neurology 2001; 56(6): 789–92
Acknowledgements
Data used in the preparation of this article were obtained from the ADNI database (www.loni.ucla.edu/ADNI). As such, the investigators within the ADNI contributed to the design and implementation of the ADNI and/or provided data but did not participate in the analysis or writing of this report. For a complete list of investigators involved in the ADNI see: http://www.loni.ucla.edu/ADNI/Data/ADNI_Authorship_List.pdf.
Data collection and sharing was funded by the ADNI (Principal Investigator: Michael Weiner; National Institutes of Health [NIH] grant U01 AG024904). The ADNI is funded by the NIA, the NIBIB and through generous contributions from the following: Pfizer, Wyeth, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Merck, AstraZeneca, Novartis, the Alzheimer’s Association, Eisai, Elan, Forest and the Institute for the Study of Aging, with participation by the FDA. Industry partnerships are coordinated through the Foundation for the National Institutes of Health. The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory of Neuro Imaging at the UCLA. Data analysis was supported in part by the following grants from the NIH: NIA R01 AG19771, P30 AG10133, NIBIB R03 EB008674 and by the Indiana Economic Development Corporation (IEDC #87884).
Dr Farlow has received research funds from Bristol-Myers Squibb, Danone, Elan, Eli Lilly, Forest, Medivation, Novartis, OctaPharma, Pfizer and Sonexa; has acted as a consultant and/or speaker for and has received honoraria from Accera, Adamas, Adlyfe, Astellas, AstraZeneca, CoMentis, Cortex, DS-Pharma (Dainippon Sumitomo Pharma), Eli Lilly, Eisai, Forest, GlaxoSmithKline, Medivation, Merck, Novartis, Noven, OctaPharma, Pfizer, QR Pharma, Sanofi-Aventis, Schering-Plough, Suven Life Sciences and Toyama; has given expert testimony for Forest; has a spouse with Eli Lilly stock; and receives royalties from Elan for a genetically engineered mouse model. Dr Epstein, Dr Saykin, Dr Gao and Shannon Risacher have no conflicts of interest that are directly relevant to the content of this study.
All authors (Dr Epstein, Dr Saykin, Shannon Risacher, Dr Gao and Dr Farlow) contributed to this study. Dr Gao, who is a professor of biostatistics at Indiana University, completed the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Epstein, N.U., Saykin, A.J., Risacher, S.L. et al. Differences in Medication Use in the Alzheimer’s Disease Neuroimaging Initiative. Drugs Aging 27, 677–686 (2010). https://doi.org/10.2165/11538260-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11538260-000000000-00000