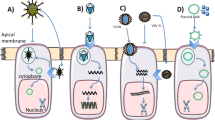
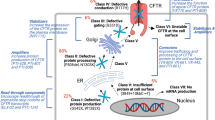
Abstract
Since the discovery of the cystic fibrosis transmembrane conductance regulator (CFTR) gene nearly 12 years ago, cystic fibrosis (CF) has become one of the most intensively investigated monogenetic disorders considered approachable by gene therapy. This has resulted in over 20 clinical trials currently under way, concluded or awaiting approval. Despite the initial promise of gene therapy for CF, and the demonstration of successful gene transfer to the nose and airways of individuals, it has not so far been as effective as initially projected. Here we discuss the rationale behind CF gene therapy and dissect the vast array of literature representing the work that ultimately brought about the current phase I/II clinical trials. In the context of human trials, we review the limitations of current vector systems for CF gene therapy. We come to the conclusion that at present none of the application methods and vector systems are able to achieve the level and persistence of CFTR gene expression in the affected epithelia of CF patients that is required for therapeutic success. We also outline the challenges that must be overcome and describe some of the novel approaches to be taken in order to attain the curative therapy that was originally envisaged for this disease.



Similar content being viewed by others
References
Dodge JA, Morison S, Lewis PA, et al. Incidence, population, and survival of cystic fibrosis in the UK, 1968-95. UK Cystic Fibrosis Survey Management Committee. Arch Dis Child 1997; 77(6): 493–6
Welsh MJ, Smith AE. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell 1993; 73(7): 1251–4
Pilewski JM, Frizzell RA. Role of CFTR in airway disease. Physiol Rev 1999; 79(1 Suppl.): S215–55
Boucher RC. Human airway ion transport. Part one. Am J Res-pir Crit Care Med 1994; 150(1): 271–81
Boucher RC. Human airway ion transport. Part two. Am J Res-pir Crit Care Med 1994; 150(2): 581–93
Zabner J, Smith JJ, Karp PH, et al. Loss of CFTR chloride channels alters salt absorption by cystic fibrosis airway epithelia in vitro. Mol Cell 1998; 2(3): 397–403
Smith JJ, Travis SM, Greenberg EP, et al. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell 1996; 85(2): 229–36
Goldman MJ, Anderson GM, Stolzenberg ED, et al. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 1997; 88(4): 553–60
Guggino WB. Cystic fibrosis and the salt controversy. Cell 1999; 96(5): 607–10
Tarran R, Grubb BR, Gatzy JT, et al. The relative roles of passive surface forces and active ion transport in the modulation of airway surface liquid volume and composition. J Gen Physiol 2001; 118(2): 223–36
Davies JC, Stern M, Dewar A, et al. CFTR gene transfer reduces the binding of Pseudomonas aeruginosa to cystic fibrosis respiratory epithelium. Am J Respir Cell Mol Biol 1997; 16(6): 657–63
Pier GB, Grout M, Zaidi TS. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc Natl Acad Sci U S A 1997; 94(22): 12088–93
Beringer PM. New approaches to optimizing antimicrobial therapy in patients with cystic fibrosis. Curr Opin Pulm Med 1999; 5(6): 371–7
Shak S, Capon DJ, Hellmiss R, et al. Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. Proc Natl Acad Sci U S A 1990; 87(23): 9188–92
Aitken ML, Burke W, McDonald G, et al. Recombinant human DNase inhalation in normal subjects and patients with cystic fibrosis. Aphase 1 study. JAMA 1992; 267(14): 1947–51
Aitken ML. Clinical trials of recombinant human DNase in cystic fibrosis patients. Monaldi Arch Chest Dis 1993; 48(6): 653–6
Hodson ME. Aerosolized dornase alfa (rhDNase) for therapy of cystic fibrosis. Am J Respir Crit Care Med 1995; 151 (3Pt2): S70–4
Ledson MJ, Wahbi Z, Convery RP, et al. Targeting of dornase alpha therapy in adult cystic fibrosis. J R Soc Med 1998; 91(7): 360–4
Bollert FG, Paton JY, Marshall TG, et al. Recombinant DNase in cystic fibrosis: a protocol for targeted introduction through n-of-1 trials. Scottish Cystic Fibrosis Group. Eur Respir J 1999; 13(1): 107–13
Dezateux C, Crighton A. Oral non-steroidal anti-inflammatory drug therapy for cystic fibrosis. Cochrane Database Syst Rev 2000; 2
Knowles MR, Church NL, Waltner WE, et al. A pilot study of aerosolized amiloride for the treatment of lung disease in cystic fibrosis. N Engl J Med 1990; 322(17): 1189–94
Hofmann T, Stutts MJ, Ziersch A, et al. Effects of topically delivered benzamil and amiloride on nasal potential difference in cystic fibrosis. Am J Respir Crit Care Med 1998; 157 (6Pt 1): 1844–9
Rodgers HC, Knox AJ. The effect of topical benzamil and amiloride on nasal potential difference in cystic fibrosis. Eur Respir J 1999; 14(3): 693–6
Jiang C, Finkbeiner WE, Widdicombe JH, et al. Altered fluid transport across airway epithelium in cystic fibrosis. Science 1993; 262(5132): 424–7
Bennett WD, Olivier KN, Zeman KL, et al. Effect of uridine 5-triphosphate plus amiloride on mucociliary clearance in adult cystic fibrosis. Am J Respir Crit Care Med 1996; 153 (6 Pt 1): 1796–801
Knowles MR, Olivier KN, Hohneker KW, et al. Pharmacologic treatment of abnormal ion transport in the airway epithelium in cystic fibrosis. Chest 1995; 107(2 Suppl.): 71S–76S
Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA [published erratum appears in Science 1989 Sep 29; 245 (4925): 1437]. Science 1989; 245(4922): 1066–73
Cole-Strauss A, Yoon K, Xiang Y, et al. Correction of the mutation responsible for sickle cell anemia by an RNA-DNA oligonucleotide. Science 1996; 273(5280): 1386–9
Kren BT, Bandyopadhyay P, Steer CJ. In vivo site-directed mutagenesis of the factor IX gene by chimeric RNA/DNA oligonucleotides. Nat Med 1998; 4(3): 285–90
Alexeev V, Igoucheva O, Domashenko A, et al. Localized in vivo genotypic and phenotypic correction of the albino mutation in skin by RNA-DNA oligonucleotide. Nat Biotechnol 2000; 18(1): 43–7
Bout A, Imler JL, Schultz H, et al. In vivo adenovirus-mediated transfer of human CFTR cDNA to rhesus monkey airway epithelium: efficacy, toxicity and safety. Gene Ther 1994; 1(6): 385–94
Zabner J, Petersen DM, Puga AP, et al. Safety and efficacy of repetitive adenovirus-mediated transfer of CFTR cDNA to airway epithelia of primates and cotton rats. Nat Genet 1994; 6(1): 75–83
Engelhardt JF, Simon RH, Yang Y, et al. Adenovirus-mediated transfer of the CFTR gene to lung of nonhuman primates: biological efficacy study. Hum Gene Ther 1993; 4(6): 759–69
Logan JJ, Bebok Z, Walker LC, et al. Cationic lipids for reporter gene and CFTR transfer to rat pulmonary epithelium. Gene Ther 1995; 2(1): 38–49
Sene C, Bout A, Imler JL, et al. Aerosol-mediated delivery of recombinant adenovirus to the airways of nonhuman primates. Hum Gene Ther 1995; 6(12): 1587–93
Flotte TR, Afione SA, Conrad C, et al. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc Natl Acad Sci U S A 1993; 90(22): 10613–7
Rosenfeld MA, Yoshimura K, Trapnell BC, et al. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell 1992; 68(1): 143–55
Yoshimura K, Rosenfeld MA, Nakamura H, et al. Expression of the human cystic fibrosis transmembrane conductance regulator gene in the mouse lung after in vivo intratracheal plas-mid-mediated gene transfer. Nucleic Acids Res 1992; 20(12): 3233–40
Brody SL, Metzger M, Danel C, et al. Acute responses of non-human primates to airway delivery of an adenovirus vector containing the human cystic fibrosis transmembrane conductance regulator cDNA. Hum Gene Ther 1994; 5(7): 821–36
Hyde SC, Gill DR, Higgins CF, et al. Correction of the ion transport defect in cystic fibrosis transgenic mice by gene therapy. Nature 1993; 362(6417): 250–5
Alton EW, Middleton PG, Caplen NJ, et al. Non-invasive lipo-some-mediated gene delivery can correct the ion transport defect in cystic fibrosis mutant mice. Nat Genet 1993; 5(2): 135–42
Alton EW, Stern M, Farley R, et al. Cationic lipid-mediated CFTR gene transfer to the lungs and nose of patients with cystic fibrosis: a double-blind placebo-controlled trial. Lancet 1999; 353(9157): 947–54
Crystal RG, McElvaney NG, Rosenfeld MA, et al. Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nat Genet 1994; 8(1): 42–51
Bellon G, Michel-Calemard L, Thouvenot D, et al. Aerosol administration of a recombinant adenovirus expressing CFTR to cystic fibrosis patients: a phase I clinical trial. Hum Gene Ther 1997; 8(1): 15–25
Zuckerman JB, Robinson CB, McCoy KS, et al. Aphase I study of adenovirus-mediated transfer of the human cystic fibrosis transmembrane conductance regulator gene to a lung segment of individuals with cystic fibrosis. Hum Gene Ther 1999; 10(18): 2973–85
Harvey BG, Leopold PL, Hackett NR, et al. Airway epithelial CFTR mRNA expression in cystic fibrosis patients after repetitive administration of a recombinant adenovirus. J Clin Invest 1999; 104(9): 1245–55
Engelhardt JF, Yankaskas JR, Ernst SA, et al. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet 1992; 2(3): 240–8
Caplen NJ, Alton EW, Middleton PG, et al. Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis [published erratum appears in Nat Med 1995 Mar; 1(3): 272]. Nat Med 1995; 1(1): 39–46
Gill DR, Southern KW, Mofford KA, et al. Aplacebo-controlled study of liposome-mediated gene transfer to the nasal epithelium of patients with cystic fibrosis. Gene Ther 1997; 4(3): 199–209
Porteous DJ, Dorin JR, McLachlan G, et al. Evidence for safety and efficacy of DOTAP cationic liposome mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Gene Ther 1997; 4(3): 210–8
Noone PG, Hohneker KW, Zhou Z, et al. Safety and biological efficacy of a lipid-CFTR complex for gene transfer in the nasal epithelium of adult patients with cystic fibrosis. Mol Ther 2000; 1(1): 105–14
Hyde SC, Southern KW, Gileadi U, et al. Repeat administration of DNA/liposomes to the nasal epithelium of patients with cystic fibrosis. Gene Ther 2000; 7(13): 1156–65
Zabner J, Couture LA, Gregory RJ, et al. Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell 1993; 75(2): 207–16
Knowles MR, Hohneker KW, Zhou Z, et al. A controlled study of adenoviral-vector-mediated gene transfer in the nasal epithelium of patients with cystic fibrosis. N Engl J Med 1995; 333(13): 823–31
Hay JG, McElvaney NG, Herena J, et al. Modification of nasal epithelial potential differences of individuals with cystic fibrosis consequent to local administration of a normal CFTR cDNA adenovirus gene transfer vector. Hum Gene Ther 1995; 6(11): 1487–96
Zabner J, Ramsey BW, Meeker DP, et al. Repeat administration of an adenovirus vector encoding cystic fibrosis transmembrane conductance regulator to the nasal epithelium of patients with cystic fibrosis. J Clin Invest 1996; 97(6): 1504–11
Wagner JA, Nepomuceno IB, Shah N, et al. Maxillary sinusitis as a surrogate model for CF gene therapy clinical trials in patients with antrostomies. J Gene Med 1999; 1(1): 13–21
Wagner JA, Reynolds T, Moran ML, et al. Efficient and persistent gene transfer of AAV-CFTR in maxillary sinus [letter]. Lancet 1998; 351(9117): 1702–3
Dorin JR, Farley R, Webb S, et al. A demonstration using mouse models that successful gene therapy for cystic fibrosis requires only partial gene correction. Gene Ther 1996; 3(9): 797–801
Gan KH, Veeze HJ, van den Ouweland AM, et al. A cystic fibrosis mutation associated with mild lung disease. N Engl J Med 1995; 333(2): 95–9
von Herrath MG, Efrat S, Oldstone MB, et al. Expression of adenoviral E3 transgenes in beta cells prevents autoimmune diabetes. Proc Natl Acad Sci U S A 1997; 94(18): 9808–13
Allen RT, Hunter 3rd WJ, Agrawal DK. Morphologic and temporal analysis of vascular smooth muscle cell apoptosis induced by c-myc and E1A. Scanning 1998; 20(8): 577–86
Robbins PD, Tahara H, Ghivizzani SC. Viral vectors for gene therapy. Trends Biotechnol 1998; 16(1): 35–40
Yang Y, Wilson JM. Clearance of adenovirus-infected hepatocytes by MHC class I-restricted CD4+ CTLs in vivo. J Immunol 1995; 155(5): 2564–70
Yang Y, Su Q, Wilson JM. Role of viral antigens in destructive cellular immune responses to adenovirus vector-transduced cells in mouse lungs. J Virol 1996; 70(10): 7209–12
Lusky M, Grave L, Dieterle A, et al. Regulation of adenovirus-mediated transgene expression by the viral E4 gene products: requirement for E4 ORF3. J Virol 1999; 73(10): 8308–19
Ramalingam R, Rafii S, Worgall S, et al. Induction of endogenous genes following infection of human endothelial cells with an El() E4(+) adenovirus gene transfer vector. J Virol 1999; 73(12): 10183–90
Whittle N. Gene therapy —the gutless approach pays off. Trends Genet 1998; 14(4): 136–7
Schiedner G, Morral N, Parks RJ, et al. Genomic DNA transfer with a high-capacity adenovirus vector results in improved in vivo gene expression and decreased toxicity [published erratum appears in Nat Genet 1998 Mar; 18 (3): 298]. Nat Genet 1998; 18(2): 180–3
Morral N, O’Neal W, Rice K, et al. Administration of helperdependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc Natl Acad Sci U S A 1999; 96(22): 12816–21
Flotte TR, Carter BJ. Adeno-associated virus vectors for gene therapy. Gene Ther 1995; 2(6): 357–62
Berns KI, Pinkerton TC, Thomas GF, et al. Detection of adenoassociated virus (AAV)-specific nucleotide sequences in DNA isolated from latently infected Detroit 6 cells. Virology 1975; 68(2): 556–60
Berns KI, Linden RM. The cryptic life style of adeno-associated virus. Bioessays 1995; 17(3): 237–45
Ponnazhagan S, Erikson D, Kearns WG, et al. Lack of site-specific integration of the recombinant adeno-associated virus 2 genomes in human cells. Hum Gene Ther 1997; 8(3): 275–84
Rutledge EA, Russell DW. Adeno-associated virus vector integration junctions. J Virol 1997; 71(11): 8429–36
Miller AD. Retrovirus packaging cells. Hum Gene Ther 1990; 1(1): 5–14
Cosset FL, Takeuchi Y, Battini JL, et al. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J Virol 1995; 69(12): 7430–6
Leigh MW, Kylander JE, Yankaskas JR, et al. Cell proliferation in bronchial epithelium and submucosal glands of cystic fibrosis patients. Am J Respir Cell Mol Biol 1995; 12(6): 605–12
Feigner PL, Gadek TR, Holm M, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A 1987; 84: 7413–7
Behr JP, Demeneix B, Loeffler JP, et al. Efficient gene transfer into mammalian primary endocrine cells with lipopolyaminecoated DNA. Proc Natl Acad Sci U S A 1989; 86(18): 6982–6
Radier JO, Koltover I, Salditt T, et al. Structure of DNA-cationic liposome complexes: DNA intercalation in multilamellar membranes in distinct interhelical packing regimes. Science 1997; 275(5301): 810–4
Wheeler JJ, Palmer L, Ossanlou M, et al. Stabilized plasmid-lipid particles: construction and characterization. Gene Ther 1999; 6(2): 271–81
Escriou V, Ciolina C, Helbling-Leclerc A, et al. Cationic lipid-mediated gene transfer: analysis of cellular uptake and nuclear import of plasmid DNA. Cell Biol Toxicol 1998; 14(2): 95–104
Zhou X, Huang L. DNA transfection mediated by cationic liposomes containing lipopolylysine: characterization and mechanism of action. Biochim Biophys Acta 1994; 1189(2): 195–203
Xu Y, Szoka Jr FC. Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry 1996; 35(18): 5616–23
Zabner J, Fasbender AJ, Moninger T, et al. Cellular and molecular barriers to gene transfer by a cationic lipid. J Biol Chem 1995; 270(32): 18997–9007
Farhood H, Serbina N, Huang L. The role of dioleoyl phosphatidylethanolamine in cationic liposome mediated gene transfer. Biochim Biophys Acta 1995; 1235(2): 289–95
88. Welsh MJ. Abnormal regulation of ion channels in cystic fibrosis epithelia. FASEB J 1990; 4(10): 2718–25
Alton EW, Currie D, Logan-Sinclair R, et al. Nasal potential difference: a clinical diagnostic test for cystic fibrosis. Eur Respir J 1990; 3(8): 922–6
Knowles MR, Paradiso AM, Boucher RC. In vivo nasal potential difference: techniques and protocols for assessing efficacy of gene transfer in cystic fibrosis. Hum Gene Ther 1995; 6(4): 445–55
Middleton PG, Geddes DM, Alton EW. Protocols for in vivo measurement of the ion transport defects in cystic fibrosis nasal epithelium. Eur Respir J 1994; 7(11): 2050–6
Stern M, Munkonge FM, Caplen NJ, et al. Quantitative fluorescence measurements of chloride secretion in native airway epithelium from CF and non-CF subjects. Gene Ther 1995; 2(10): 766–74
Wagner JA, Messner AH, Moran ML, et al. Safety and biological efficacy of an adeno-associated virus vector-cystic fibrosis transmembrane regulator (AAV-CFTR) in the cystic fibrosis maxillary sinus. Laryngoscope 1999; 109 (2 Pt 1): 266–74
Zabner J, Cheng SH, Meeker D, et al. Comparison of DNA-lipid complexes and DNA alone for gene transfer to cystic fibrosis airway epithelia in vivo. J Clin Invest 1997; 100(6): 1529–37
Joseph PM, O’Sullivan BP, Lapey A, et al. Aerosol and lobar administration of a recombinant adenovirus to individuals with cystic fibrosis. I. Methods, safety, and clinical implications. Hum Gene Ther 2001; 12(11): 1369–82
Perricone MA, Morris JE, Pavelka K, et al. Aerosol and lobar administration of a recombinant adenovirus to individuals with cystic fibrosis. II. Transfection efficiency in airway epithelium. Hum Gene Ther 2001; 12(11): 1383–94
Harvey BG, Hackett NR, El-Sawy T, et al. Variability of human systemic humoral immune responses to adenovirus gene transfer vectors administered to different organs. J Virol 1999; 73(8): 6729–42
Moss RB, King VV. Management of sinusitis in cystic fibrosis by endoscopie surgery and serial antimicrobial lavage. Reduction in recurrence requiring surgery. Arch Otolaryngol Head Neck Surg 1995; 121(5): 566–72
Wine JJ, King VV, Lewiston NJ. Method for rapid evaluation of topically applied agents to cystic fibrosis airways. Am J Physiol 1991; 261 (2 Pt 1): L218–21
Chadwick SL, Kingston HD, Stern M, et al. Safety of a single aerosol administration of escalating doses of the cationic lipid GL-67/DOPE/DMPE-PEG5000 formulation to the lungs of normal volunteers. Gene Ther 1997; 4(9): 937–42
Ruiz FE, Clancy JP, Perricone MA, et al. A clinical inflammatory syndrome attributable to aerosolized lipid-DNA administration in cystic fibrosis. Hum Gene Ther 2001; 12(7): 751–61
Scheule RK, St George JA, Bagley RG, et al. Basis of pulmonary toxicity associated with cationic lipid-mediated gene transfer to the mammalian lung. Hum Gene Ther 1997; 8(6): 689–707
Noone PG, Knowles MR. Interpretation of CF gene therapy trial results. Mol Ther 2000; 2(1): 4
McDonald RJ, Liggitt HD, Roche L, et al. Aerosol delivery of lipid: DNA complexes to lungs of rhesus monkeys. Pharm Res 1998; 15(5): 671–9
Johnson LG, Boyles SE, Wilson J, et al. Normalization of raised sodium absorption and raised calcium-mediated chloride secretion by adenovirus-mediated expression of cystic fibrosis transmembrane conductance regulator in primary human cystic fibrosis airway epithelial cells. J Clin Invest 1995; 95(3): 1377–82
Simon RH, Engelhardt JF, Yang Y, et al. Adenovirus-mediated transfer of the CFTR gene to lung of nonhuman primates: toxicity study. Hum Gene Ther 1993; 4(6): 771–80
Yei S, Mittereder N, Tang K, et al. Adenovirus-mediated gene transfer for cystic fibrosis: quantitative evaluation of repeated in vivo vector administration to the lung. Gene Ther 1994; 1(3): 192–200
Harvey BG, Hackett NR, Ely S, et al. Host responses and persistence of vector genome following intrabronchial administration of an E1(−) E3(−) adenovirus gene transfer vector to normal individuals. Mol Ther 2001; 3(2): 206–15
Brenner M. Reports of adenovector ‘death’ are greatly exaggerated. Mol Ther 2000; 1(3): 205
Li S, Tseng WC, Stolz DB, et al. Dynamic changes in the characteristics of cationic lipidic vectors after exposure to mouse serum: implications for intravenous lipofection. Gene Ther 1999; 6(4): 585–94
Freimark BD, Blezinger HP, Florack VJ, et al. Cationic lipids enhance cytokine and cell influx levels in the lung following administration of plasmid: cationic lipid complexes. J Immunol 1998; 160(9): 4580–6
Yew NS, Zhao H, Wu IH, et al. Reduced inflammatory response to plasmid DNA vectors by elimination and inhibition of immunostimulatory CpG motifs. Mol Ther 2000; 1(3): 255–62
Krieg AM. An innate immune defense mechanism based on the recognition of CpG motifs in microbial DNA. J Lab Clin Med 1996; 128(2): 128–33
Krieg AM. Direct immunologic activities of CpG DNA and implications for gene therapy. J Gene Med 1999; 1(1): 56–63
Yew NS, Wang KX, Przybylska M, et al. Contribution of plasmid DNA to inflammation in the lung after administration of cationic lipid: pDNA complexes. Hum Gene Ther 1999; 10(2): 223–34
Norman J, Denham W, Denham D, et al. Liposome-mediated, nonviral gene transfer induces a systemic inflammatory response which can exacerbate pre-existing inflammation. Gene Ther 2000; 7(16): 1425–30
McLachlan G, Stevenson BJ, Davidson DJ, et al. Bacterial DNA is implicated in the inflammatory response to delivery of DNA/DOTAP to mouse lungs. Gene Ther 2000; 7(5): 384–92
Bigger BW, Tolmachov O, Collombet JM, et al. An araC controlled bacterial cre expression system to produce DNA minicircle vectors for nuclear and mitochondrial gene therapy. J Biol Chem 2001; 276(25): 23018–27
Stern M, Caplen NJ, Browning JE, et al. The effect of mucolytic agents on gene transfer across a CF sputum barrier in vitro. Gene Ther 1998; 5(1): 91–8
Pickles RJ, McCarty D, Matsui H, et al. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J Virol 1998; 72(7): 6014–23
Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol 1998; 72(2): 1438–45
Curiel DT. Strategies to adapt adenoviral vectors for targeted delivery. Ann N Y Acad Sci 1999; 886 158–71
Zabner J, Chillon M, Grünst T, et al. A chimeric type 2 adenovirus vector with a type 17 fiber enhances gene transfer to human airway epithelia. J Virol 1999; 73(10): 8689–95
Jost PJ, Harbottle RP, Knight A, et al. A novel peptide, THALWHT, for the targeting of human airway epithelia. FEBS Lett 2001; 489(2-3): 263–9
Link CJ. Adenoviral vectors go retro. Nat Biotechnol 2000; 18(2): 150–1
Goldman MJ, Lee PS, Yang JS, et al. Lentiviral vectors for gene therapy of cystic fibrosis. Hum Gene Ther 1997; 8(18): 2261–8
Lewis P, Hensel M, Emerman M. Human immunodeficiency virus infection of cells arrested in the cell cycle [published erratum appears in EMBO J 1992 Nov; 11(11): 4249]. EMBO J 1992; 11(8): 3053–8
Park F, Ohashi K, Chiu W, et al. Efficient lentiviral transduction of liver requires cell cycling in vivo. Nat Genet 2000; 24(1): 49–52
Naldini L, Blomer U, Gage FH, et al. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci U S A 1996; 93(21): 11382–8
Wang G, Slepushkin V, Zabner J, et al. Feline immunodeficiency virus vectors persistently transduce nondividing airway epithelia and correct the cystic fibrosis defect. J Clin Invest 1999; 104(11): R55–62
Zabner J, Seiler M, Walters R, et al. Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. J Virol 2000; 74(8): 3852–8
Halbert CL, Allen JM, Miller AD. Adeno-associated virus type 6 (AAV6) vectors mediate efficient transduction of airway epithelial cells in mouse lungs compared to that of AAV2 vectors. J Virol 2001; 75(14): 6615–24
Yonemitsu Y, Kitson C, Ferrari S, et al. Efficient gene transfer to airway epithelium using recombinant Sendai virus. Nat Biotechnol 2000; 18(9): 970–3
Wang G, Zabner J, Deering C, et al. Increasing epithelial junction permeability enhances gene transfer to airway epithelia In vivo. Am J Respir Cell Mol Biol 2000; 22(2): 129–38
Parsons DW, Grubb BR, Johnson LG, et al. Enhanced in vivo airway gene transfer via transient modification of host barrier properties with a surface-active agent. Hum Gene Ther 1998; 9(18): 2661–72
Coyne CB, Kelly MM, Boucher RC, et al. Enhanced epithelial gene transfer by modulation of tight junctions with sodium caprate. Am J Respir Cell Mol Biol 2000; 23(5): 602–9
Kitson C, Angel B, Judd D, et al. The extra-and intracellular barriers to lipid and adenovirus-mediated pulmonary gene transfer in native sheep airway epithelium. Gene Ther 1999; 6(4): 534–46
Harbottle RP, Cooper RG, Hart SL, et al. An RGD-oligolysine peptide: a prototype construct for integrin-mediated gene delivery. Hum Gene Ther 1998; 9(7): 1037–47
Colin M, Harbottle RP, Knight A, et al. Liposomes enhance delivery and expression of an RGD-oligolysine gene transfer vector in human tracheal cells. Gene Ther 1998; 5(11): 1488–98
Boussif O, Lezoualc’h F, Zanta MA, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A 1995; 92(16): 7297–301
Aronsohn AI, Hughes JA. Nuclear localization signal peptides enhance cationic liposome-mediated gene therapy. J Drug Target 1998; 5(3): 163–9
Zanta MA, Belguise-Valladier P, Behr JP. Gene delivery: a single nuclear localization signal peptide is sufficient to carry DNA to the cell nucleus. Proc Natl Acad Sci U S A 1999; 96(l): 91–6
Branden LJ, Mohamed AJ, Smith CI. A peptide nucleic acid-nuclear localization signal fusion that mediates nuclear transport of DNA. Nat Biotechnol 1999; 17(8): 784–7
Henning KA, Novotny EA, Compton ST, et al. Human artificial chromosomes generated by modification of a yeast artificial chromosome containing both human alpha satellite and single-copy DNA sequences. Proc Natl Acad Sci USA 1999;96(2): 592–7
Simpson K, McGuigan A, Huxley C. Stable episomal maintenance of yeast artificial chromosomes in human cells. Mol Cell Biol 1996; 16(9): 5117–26
Zepeda M, Wilson JM. Neonatal cotton rats do not exhibit destructive immune responses to adenoviral vectors. Gene Ther 1996; 3(11): 973–9
Rubenstein RC, McVeigh U, Flotte TR, et al. CFTR gene transduction in neonatal rabbits using an adeno-associated virus (AAV) vector. Gene Ther 1997; 4(5): 384–92
Huard J, Lochmuller H, Acsadi G, et al. Differential short-term transduction efficiency of adult versus newborn mouse tissues by adenoviral recombinants. Exp Mol Pathol 1995; 62(2): 131–43
Coutelle C, Douar AM, Colledge WH, et al. The challenge of fetal gene therapy. Nat Med 1995; 1(9): 864–6
Sekhon HS, Larson JE. In utero gene transfer into the pulmonary epithelium. Nat Med 1995; 1(11): 1201–3
Douar AM, Adebakin S, Themis M, et al. Foetal gene delivery in mice by intra-amniotic administration of retroviral producer cells and adenovirus. Gene Ther 1997; 4(9): 883–90
Larsson JE, Morrow SL, Happel L, et al. Reversal of cystic fibrosis phenotype in mice by gene therapy in utero. Lancet 1997; 349(9052): 619–20
Alton E, Smith S, Geddes D. Gene therapy for cystic fibrosis. Lancet 1997; 349(9060): 1249–50
Colledge WH. Gene therapy for cystic fibrosis. Lancet 1997; 349(9060): 1249
Acknowledgements
The work of the Cystic Fibrosis Gene Therapy Research Group is supported by the Medical Research Council, The March of Dimes Birth Defects Foundation and Vaincre la Mucoviscidose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bigger, B., Coutelle, C. Perspectives on Gene Therapy for Cystic Fibrosis Airway Disease. BioDrugs 15, 615–534 (2001). https://doi.org/10.2165/00063030-200115090-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00063030-200115090-00006