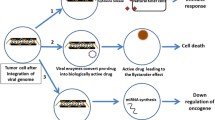
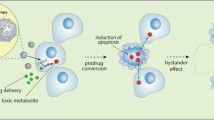
Summary
Tumour development is a disease of both somatic and genetic origin. It originates from a combination of oncogene and tumour suppressor alterations that force the cell into unprogrammed proliferation. It is reasonable to think that a genetic disease could be cured by gene therapy, and several strategies along this line are presently being employed. The use of retroviruses to carry ‘suicide’ genes has been the most successful approach to date for the treatment of neurological malignancies. The use of adenoviruses with deletions in the E1B region opens a new and elegant possibility for the destruction of tumours deficient in the p53 suppressor gene. Strategies based on the inhibition of angiogenesis are being developed, and those based on blockade of inducers of angiogenesis have given encouraging results in experimental animal models. However, gene therapy has not yet been able to permanently cure a human genetic disease, thereby creating a certain degree of caution among scientists and clinicians.
Similar content being viewed by others
References
Fults D, Brockmeyrer D, Tullous MW, et al. p53 mutation and loss of heterozygosity on chromosomes 17 and 10 during human astrocytoma progression. Cancer Res 1992; 52: 674–9
Brodeur GM, Seeger RC, Schwab M, et al. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science 1984; 224: 1121–4
Brodeur GM, Hayes FA, Green AA, et al. Consistent N-myc copy number in simultaneous or consecutive neuroblastoma samples from sixty individual patients. Cancer Res 1987; 47: 4248–53
Libermann TA, Nusbaum HR, Razon N, et al. Amplification enhanced expressions and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature 1985; 313: 144–7
Maiden IT, Novac V, Kaye AH, et al. Selective amplification on the cytoplasmic domain of the epidermal growth factor receptor gene in glioblastoma multiforme. Cancer Res 1988; 48: 2711–4
Frankel RH, Bayona W, Koslow M, et al. p53 mutations in human malignant gliomas: comparison of loss of heterozygosity with mutation frequency. Cancer Res 1992; 52: 1427–33
Imamura J, Bartram CR, Berthold F, et al. Mutation of the p53 gene in neuroblastoma and its relationship with N-myc amplification. Cancer Res 1993; 53: 4053–8
Sidransky D, Mikkelsen T, Schwechheimer K, et al. Clonal expansion of p53 mutant cells is associated with brain tumour progression. Nature 1992; 348: 747–9
Del Arco A, García J, Arribas C, et al. Timing of p53 mutations during astrocytoma tumorigenesis. Hum Mol Genet 1993; 2: 1687–90
Ahmed Rasheed BK, McLendon RE, Herndon JE, et al. Alterations of the TP53 gene in human gliomas. Cancer Res 1994; 54: 1324–30
Yahanda AM, Bruner JM, Donehower LA, et al. Astrocytes derived from p53-deficient mice provide a multistep in vitro model for development of malignant gliomas. Mol Cell Biol 1995; 15: 4249–59
Moolten FL. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res 1986; 46: 5276–81
Moolten FL, Wells JM. Curability of tumors bearing herpes thymidine kinase genes transfected by retroviral vectors. J Natl Cancer Inst 1990; 82: 297–300
Moolten FL, Wells JM, Heyman RA, et al. Lymphoma regression induced by ganciclovir in mice bearing a herpes thymidine kinase transgene. Hum Gene Ther 1990; 1: 125–35
Short MP, Choi BC, Lee JK, et al. Gene delivery to glioma cells in rat brain by grafting of a retrovirus packaging cell line. J Neurosci Res 1990; 27: 427–433
Takamiya Y, Short MP, Ezzeddine ZD, et al. Gene therapy of malignant brain tumors: a rat glioma line bearing the herpes simplex virus type 1 thymidine kinase gene and wild type retrovirus kills other tumor cells. J Neurosci Res 1992; 33: 493–503
Culver KW, Wallbridge S, Ishii H, et al. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science 1992; 256: 1550–2
Ram Z, Culver KW, Walbridge S, et al. In situ retroviral-mediated gene transfer for the treatment of brain tumors in rats. Cancer Res 1993; 53: 83–8
Izquierdo M, Cortés M, de Felipe P, et al. Long-term rat survival after malignant brain tumor regression by retroviral gene therapy. Gene Ther 1995; 2: 66–9
Culver KW, Van Gilder J, Link CJ, et al. Gene therapy for the treatment of malignant brain tumors with in vivo tumor transduction with herpes simplex thymidine kinase gene/ganciclovir system. Hum Gene Ther 1994; 5: 343–79
Izquierdo M, Martín V, de Felipe P, et al. Human malignant brain tumor response to herpes simplex thymidine kinase (HSVtk)/ganciclovir gene therapy. Gene Ther 1996; 3: 491–5
Roth JA, Cristiano RJ. Gene therapy for cancer: what have we done and where are we going? J Natl Cancer Inst 1997; 89: 21–39
Akli S, Caillaud C, Vigne E, et al. Transfer of a foreign gene into the brain using adenovirus vectors. Nature Genet 1993; 3: 224–8
Le Gal La Salle G, Robert JJ, Berrard S, et al. An adenovirus vector for gene transfer into neurons and glia in the brain. Science 1993; 259: 988–90
Neve RL. Adenovirus vectors enter the brain. Trends Neurosci 1993; 16: 251–3
Lowenstein PR, Wilkinson GWG, Castro MG, et al. Non-neurotropic adenovirus: a vector for gene transfer to the brain and possible gene therapy of neurological disorders. In: Latchman D, editor. Genetic manipulation of the nervous system. New York: Academic Press, 1995: 1–39
Kramm CM, Sena-Esteves M, Barnett M, et al. Gene therapy for brain tumors. Brain Pathol 1995; 5: 345–81
Shine HD, Woo SLC. Adenovirus-mediated gene therapy of tumors in the central nervous system. In: Latchman D, editor. Genetic manipulation of the nervous system. New York: Academic Press, 1995: 53–71
Viola JJ, Ram Z, Walbridge S, et al. Adenovirally mediated gene transfer into experimental solid brain tumors and leptomeningeal cancer cells. J Neurosurg 1995; 82: 70–6
Boviatsis EJ, Chase M, Wei MX, et al. Gene transfer into experimental brain tumors mediated by adenovirus herpes simplex virus and retrovirus vectors. Hum Gene Ther 1994; 5: 183–91
Chen SH, Shine HD, Goodman JC, et al. Gene therapy for brain tumors: regression of experimental gliomas by adenovirus-mediated gene transfer in vivo. Proc Natl Acad Sci USA 1994; 91: 3054–7
Perez-Cruet MJ, Tarsk TW, Chen SH, et al. Adenovirus-mediated gene therapy of experimental gliomas. J Neurosci Res 1994; 39: 506–11
Colak A, Goodman JC, Chen S, et al. Adenovirus-mediated gene therapy for experimental spinal cord tumors: tumoricidal efficacy and functional outcome. Brain Res 1995; 691: 76–82
Colak A, Goodman JC, Chen S, et al. Adenovirus-mediated gene therapy in an experimental model of breast cancer metastatic to the brain. Hum Gene Ther 1995; 6: 1317–22
Goodman JC, Trask TW, Chen SH, et al. Adenoviral-mediated thymidine kinase gene transfer into the primate brain followed by systemic ganciclovir: pathologic, radiologic and molecular studies. Hum Gene Ther 1996; 7: 1241–50
Maron A, Havaux N, Le Roux A, et al. Differential toxicity of ganciclovir for rat neurons and astrocytes in primary culture following adenovirus-mediated transfer of HSVtk gene. Gene Ther 1997; 4: 25–31
Doran SE, Rossler BJ, Hartman JW, et al. Adenovirus-mediated in vivo gene transfer into the central nervous system of a non-human primate. Clin Neurosurg 1994; 41: 242–57
Bischoff JR, Kirn DH, Williams A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 1996; 274: 373–6
Latchman DS. Herpes simplex virus vectors for gene therapy. Mol Biotechnol 1994; 2: 179–95
Mineta T, Rabkin SAD, Martuza RL. Treatment of malignant gliomas using ganciclovir-hypersensitive ribonucleotide reductase-deficient herpes simplex viral mutant. Cancer Res 1994; 54: 3963–6
Randazzo BP, Kesari S, Gesser RM, et al. Treatment of experimental intracranial murine melanoma with a neuroattenuated herpes simplex virus 1 mutant. Virology 1995; 211: 94–101
Naldini L, Blömer U, Gage FH, et al. Efficient transfer integration and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA 1996; 93: 11382–8
Mizuno M, Yoshida J, Sugita K, et al. Growth inhibition of glioma cells transfected with the human β-interferon gene by liposomes coupled with a monoclonal antibody. Cancer Res 1990; 50: 7826–9
Denekamp J. Angiogenesis, neovascular proliferation and vascular pathophysiology as targets for cancer therapy. Br J Radiol 1993; 66: 181–96
Fidler IJ, Ellis LM. The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell 1994; 79: 185–8
Baillie CT, Winslet MC, Bradley NJ. Tumour vasculature — a potential therapeutic target. Br J Cancer 1995; 72: 257–67
Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996; 86: 353–64
Fan T-PD, Jaggar R, Bicknell R. Controlling the vasculature: angiogenesis, anti-angiogenesis and vascular targeting of gene therapy. Trends Pharmacol Sci 1995; 16: 57–66
Kerbel RS. Inhibition of tumor angiogenesis as a strategy to circumvent acquired resistance to anti-cancer therapeutic agents. Bioessays 1991; 13: 31–6
Christofori G. The role of fibroblast growth factors in tumour progression and angiogenesis. In: Bicknell R, Lewis CE, Ferrara N, editors. Tumor angiogenesis. Oxford: Oxford University Press, 1997: 201–37
Brown LF, Detmar M, Claffey K, et al. Vascular permeability factor/vascular endothelial growth factor: a multifunctional angiogenic cytokine. In: Goldberg ID, Rosen E, editors. Regulation of angiogenesis. Berlin: Birkhauser Verlag, 1997: 233–69
Cheng S-Y, Huang HJS, Nagane M, et al. Suppression of glioblastoma angiogenicity and tumorigenicity by inhibition of endogenous expression of vascular endothelial growth factor. Proc Natl Acad Sci USA 1996; 93: 8502–7
Rastinejad F, Polverini PJ, Bouck NP. Regulation of the activity of a new inhibitor of angiogenesis by a cancer suppressor gene. Cell 1989; 56: 345–55
Van Meir EG, Polverini PJ, Chazin VR, et al. Release of an inhibitor of angiogenesis upon induction of wild type p53 expression in glioblastoma cells. Nature Genet 1994; 8: 171–6
Good DJ, Polverini PJ, Rastinejad F, et al. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci USA 1990; 87: 6624–8
Parangi S, O’Reilly M, Christofori G, et al. Antiangiogenic therapy of transgenic mice impairs Je novo tumor growth. Proc Natl Acad Sci USA 1996; 93: 2002–7
Freeman S, Abboud C, Whartenby K, et al. The ‘bystander effect’: tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res 1993; 53: 5274–83
Ishii H, Agbaria R, Hirano H, et al. Mechanism of ‘bystander effect’ killing in the herpes simplex thymidine kinase gene-modified tumor system. J Cell Biochem 1994; Suppl. 11A: 226
Barba D, Hardin J, Sadelaine M, et al. Development of antitumor immunity following thymidine kinase-mediated killing of experimental brain tumors. Proc Natl Acad Sci USA 1994; 91: 4348–52
Elshami AA, Saavedra A, Zhang H, et al. Gap junctions play a role in the ‘bystander effect’ of the herpes simplex virus thymidine kinase/ganciclovir system in vitro. Gene Ther 1996; 3: 85–92
Izquierdo M, Cortés ML, Martín V, et al. Implications of the size of glioblastoma on its curability. Acta Neurochir (Wien) 1997; 68: 111–7
Wagner E, Zatloukal K, Cotten M, et al. Coupling of adenovirus to transferrin-polylysine/DNA complexes greatly enhances receptor-mediated gene delivery and expression of transfected genes. Proc Natl Acad Sci USA 1992; 89: 6099–103
von Rüden T, Stingl L, Cotten M, et al. Generation of high-titer retroviral vectors following receptor-mediated adenovirus-augmented transfection. Biotechniques 1995; 18: 484–9
Morgan RA, Couture L, Elroy-Stein O, et al. Retroviral vectors containing putative internal ribosome entry sites: development of a polycistronic gene transfer system and applications to human gene therapy. Nucleic Acids Res 1992; 20: 1293–9
Hertel LW, Boder GB, Kroin JS, et al. Evaluation of the antitumor activity of gemcitabine (2′,2′-difluoro-2′-deoxycytidine). Cancer Res 1990; 50: 4417–22
Chottiner EG, Shewach DS, Datta NS, et al. Cloning and expression of human deoxycytidine kinase cDNA. Proc Natl Acad Sci USA 1991; 88: 1531–5
Manome Y, Wen P, Y, et al. Viral vector transduction of the human deoxycytidine kinase cDNA sensitizes glioma cells to the cytotoxic effects of cytosine arabinoside in vitro and in vivo. Nature Med 1996; 2: 567–73
Hapke DM, Stegmann APA, Mitchell BS. Retroviral transfer of deoxycytidine kinase into tumor cell lines enhances nucleo-side toxicity. Cancer Res 1996; 56: 2343–7
Kufe DW, Major PP, Egan EM, et al. Correlation of cytotoxicity with incorporation of ara-C into DNA. J Biol Chem 1980; 255: 8997–9000
Tamura T, Aoyama A, Inoue T, et al. Tissue-specific in vitro transcription from the mouse myelin basic protein promoter. Mol Cell Biol 1989; 3122-6
Miura M, Tamura T, Aoyama A, et al. The promoter elements of the mouse myelin basic protein gene function efficiently in NG108-15 neuronal/glial cells. Gene 1989; 75: 31–8
Tamura T, Sumita K, Mikoshiba K. Sequences involved in brain-specific in vitro transcription from the core promoter of the mouse myelin basic protein gene. Biochim Biophys Acta 1991; 1129: 83–6
Ikenaka K, Nakahira K, Nakajima K, et al. Detection of brain-specific gene expression in brain cells in primary culture: a novel promoter assay based on the use of a retrovirus vector. New Biol 1992; 4: 53–60
Miyao Y, Shimizu K, Moriuchi S, et al. Selective expression of foreign genes in glioma cells: use of the mouse myelin basic protein gene promoter to direct toxic gene expression. J Neurosci Res 1993; 36: 472–9
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Izquierdo, M.L. An Overview of Gene Therapy Approaches to Neurological Malignancies. BioDrugs 9, 337–349 (1998). https://doi.org/10.2165/00063030-199809040-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00063030-199809040-00006