Summary
Abstract
Salmeterol/fluticasone propionate, administered twice daily via a multidose dry powder inhaler (Seretide/Advair Diskus®, Seretide Accuhaler®) or metered-dose hydrofluoroalkane (chlorofluorocarbon-free) inhaler (Seretide Evohaler®), is a combination of the long-acting β2-adrenoceptor agonist (β2-agonist) [LABA] salmeterol and the corticosteroid fluticasone propionate.
Maintenance therapy with combined salmeterol/fluticasone propionate is at least as effective in improving lung function and symptoms and is as well tolerated in patients with asthma as concurrent salmeterol plus fluticasone propionate. In patients previously receiving as-required short-acting β2-agonists (SABAs) or inhaled corticosteroids, salmeterol/fluticasone propionate was significantly more effective in providing asthma control than fluticasone propionate and in improving lung function and asthma symptoms than inhaled corticosteroids (at equivalent or higher dosages), salmeterol or montelukast (as monotherapy or in combination with fluticasone propionate). Salmeterol/fluticasone propionate was more effective in improving asthma symptoms than adjusted-dose budesonide/formoterol in patients with uncontrolled asthma despite treatment with inhaled corticosteroids with or without a LABA in a well designed 1-year study. In pharmacoeconomic analyses, salmeterol/fluticasone propionate compared favourably with inhaled corticosteroids and mono- or combination therapy with oral montelukast. Salmeterol/fluticasone propionate is, therefore, an effective, well tolerated and cost-effective option for the maintenance treatment of patients with asthma.
Pharmacological Properties
Salmeterol protects against bronchoconstriction caused by a variety of agents or exercise in patients with asthma and possesses a range of anti-inflammatory actions. Fluticasone propionate inhibits the number and function of a wide range of proinflammatory cells in the bronchial epithelium and submucosa and reduces bronchial hyperresponsiveness to various stimuli in patients with asthma. In vitro data suggest additive or synergistic effects between LABAs and corticosteroids that may benefit patients with asthma.
The pharmacokinetics of salmeterol and fluticasone propionate administered concomitantly using the same inhaler are generally similar to those of the two agents administered separately. The absolute bioavailability of fluticasone propionate is 10–30% of the inhaled dose. Salmeterol is extensively metabolised by hydroxylation, whereas fluticasone propionate is metabolised by the cytochrome P450 (CYP) isoenzyme CYP3A4 to an inactive metabolite. Drug interactions between fluticasone propionate and potent CYP3A4 inhibitors may occur.
Therapeutic Efficacy
Combined salmeterol/fluticasone propionate was as least as effective in improving lung function and asthma symptoms as the two agents administered using separate inhalers in adults, adolescents and children (aged 4–11 years).
Significantly more patients receiving twice-daily salmeterol/fluticasone propionate than those receiving twice-daily fluticasone propionate achieved totally controlled asthma in a large 1-year study in patients with asthma of varying severity. Moreover, in 12- or 24-week studies in asthmatic patients previously receiving as-required SABAs or inhaled corticosteroids, improvements from baseline in lung function and asthma symptoms were significantly greater with salmeterol/fluticasone propionate than with monotherapy with inhaled corticosteroids, salmeterol or montelukast, or combination therapy with montelukast plus fluticasone propionate. In several studies, this was achieved at substantially lower corticosteroid dosages. In addition, salmeterol/fluticasone propionate provided more effective protection against exercise-induced asthma than fluticasone propionate. In a well designed 1-year trial in patients with uncontrolled asthma despite receiving inhaled corticosteroids with or without a LABA, salmeterol/fluticasone propionate was more effective in improving asthma symptoms and preventing exacerbations than adjusted-dose budesonide/formoterol.
Twice-daily salmeterol/fluticasone propionate produced clinically meaningful improvements from baseline in asthma-related quality of life scores.
Tolerability
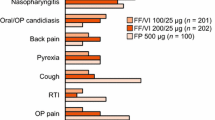
Salmeterol/fluticasone propionate was well tolerated in patients with asthma in trials of 4–52 weeks’ duration. The most frequently occurring adverse events were headache, throat irritation/cough, hoarseness/dysphonia and candidiasis. No significant differences in markers of systemic glucocorticoid activity were seen between patients receiving concurrent or combined salmeterol/fluticasone propionate 50μg/100μg or 50μg/500μg twice daily in 12- or 28-week studies, or between patients receiving salmeterol/fluticasone propionate 50μg/250μg or 50μg/500μg twice daily or the same nominal dosage of fluticasone propionate.
Pharmacoeconomic Studies
In pharmacoeconomic analyses from a healthcare payer perspective, salmeterol/fluticasone propionate was cost effective. It was associated with favourable incremental function- and symptom-based cost-effectiveness ratios relative to monotherapy with inhaled corticosteroids or oral montelukast in the UK, US and Sweden. Salmeterol/fluticasone propionate was cost saving relative to combination therapy with montelukast plus fluticasone propionate with regard to the cost per successfully treated patient in the US and with regard to the cost per successfully treated week, symptom-free day, symptom-free night and episode-free day in The Netherlands.






Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Bousquet J, Jeffery PK, Busse WW, et al. Asthma: from bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 2000 May; 161(5): 1720–45
Adkins JC, McTavish D. Salmeterol: a review of its pharmacological properties and clinical efficacy in the management of children with asthma. Drugs 1997; 54(2): 331–54
Jarvis B, Faulds D. Inhaled fluticasone propionate: a review of its therapeutic efficacy at dosages ≤500 μg/day in adults and adolescents with mild to moderate asthma. Drugs 1999; 57(5): 769–803
Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting β2-agonists and corticosteroids. Eur Respir J 2002 Jan; 19(1): 182–91
Chung KF, Adcock IM. Combination therapy of long-acting β2-adrenoceptor agonists and corticosteroids for asthma. Treat Respir Med 2004; 3(5): 279–89
Kirby S, Falcoz C, Daniel MJ, et al. Salmeterol and fluticasone propionate given as a combination: lack of systemic pharmacodynamic and pharmacokinetic interactions. Eur J Clin Pharmacol 2001; 56: 781–91
Glaxo Wellcome UK Limited. Seretide 100, 250, 500 Accuhaler: summary of product characteristics. Uxbridge, UK: Glaxo Wellcome UK Limited, 2004 Dec
Glaxo Wellcome UK Limited. Seretide 50, 125, 250 Evohaler: summary of product characteristics. Uxbridge, UK: Glaxo Wellcome UK Limited, 2004 Dec
GlaxoSmithKline. Advair Diskus 100/50, 250/50, 500/50 prescribing information. Research Triangle Park (NC): GlaxoSmithKline, 2004 Sep
Daley-Yates PT, Harker AJ, Taylor S, et al. Plasma protein binding (PPB) of corticosteroids (CS): reappraisal of its significance in systemic pharmacological activity [abstract no. 13]. J Allergy Clin Immunol 2005; 115 (2 Suppl. 1): S4
Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma controL study. Am J Respir Crit Care Med 2004 Oct; 170(8): 836–44
McCarthy TP, Greening AP, Holgate SK, et al. Salmeterol/fluticasone propionate combination (SFC) is more effective than beclometasone dipropionate (BDP) in patients not well controlled on bronchodilators alone [abstract]. Am J Respir Crit Care Med 2002 Apr; 165(8 Pt 2): A566
Murray J, Rosenthal R, Somerville L, et al. Fluticasone propionate and salmeterol administered via Diskus compared with salmeterol or fluticasone propionate alone in patients suboptimally controlled with short-acting β2-agonists. Ann Allergy Asthma Immunol 2004 Oct; 93(4): 351–9
Nelson HS, Wolfe JD, Gross G, et al. Efficacy and safety of fluticasone propionate 44 μg/salmeterol 21 μg administered in a hydrofluoroalkane metered-dose inhaler as an initial asthma maintenance treatment. Ann Allergy Asthma Immunol 2003 Sep; 91(3): 263–9
Strand AM, Luckow A. Initiation of maintenance treatment of persistent asthma: salmeterol/fluticasone propionate combination treatment is more effective than inhaled steroid alone. Danish INitiative for Asthma treatment. Respir Med 2004 Oct; 98(10): 1008–15
Bergmann K-C, Lindemann L, Braun R, et al. Salmeterol/fluticasone propionate (50/250 μg) combination is superior to double dose fluticasone (500 μg) for the treatment of symptomatic moderate asthma. Swiss Med Wkly 2004 Jan 24; 134(3–4): 50–8
Jenkins C, Woolcock AJ, Saarelainen P, et al. Salmeterol/fluticasone propionate combination therapy 50/250 μg twice daily is more effective than budesonide 800 μg twice daily in treating moderate to severe asthma. Respir Med 2000; 94: 715–23
Johansson G, McIvor RA, D’Ambrosio FP, et al. Comparison of salmeterol/fluticasone propionate combination with budesonide in patients with mild-to-moderate asthma. Clin Drug Invest 2001; 21(9): 633–42
Shapiro G, Lumry W, Wolfe J, et al. Combined salmeterol 50 μg and fluticasone propionate 250 μg in the Diskus device for the treatment of asthma. Am J Respir Crit Care Med 2000; 161: 527–34
Busse W, Koenig SM, Oppenheimer J, et al. Steroid-sparing effects of fluticasone propionate 100 μg and salmeterol 50 μg administered twice daily in a single product in patients previously controlled with fluticasone propionate 250 μg administered twice daily. J Allergy Clin Immunol 2003 Jan; 111(1): 57–65
Kavuru M, Melamed J, Gross G, et al. Salmeterol and fluticasone propionate combined in a new powder inhalation device for the treatment of asthma: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol 2000; 105(6 Pt 1): 1108–16
Pearlman DS, Peden D, Condemi JJ, et al. Efficacy and safety of fluticasone propionate/salmeterol HFA 134A MDI in patients with mild-to-moderate persistent asthma. J Asthma 2004; 41(8): 797–806
Weiler JM, Nathan RA, Rupp NT, et al. Effect of fluticasone/salmeterol administered via a single device on exercise-induced bronchospasm in patients with persistent asthma. Ann Allergy Asthma Immunol 2005 Jan; 94(1): 65–72
Dorinsky P, Stauffer J, Waitkus-Edwards K, et al. “Stepping down” from fluticasone propionate/salmeterol 100/50mcg Diskus results in loss of asthma control [abstract no. P1979]. Eur Respir J 2004 Sep; 24 Suppl. 48: 309s
Calhoun WJ, Nelson HS, Nathan RA, et al. Comparison of fluticasone propionate-salmeterol combination therapy and montelukast in patients who are symptomatic on short-acting β2-agonists alone. Am J Respir Crit Care Med 2001 Sep 1; 164: 759–63
Pearlman DS, White MV, Lieberman AK, et al. Fluticasone propionate/salmeterol combination compared with montelukast for the treatment of persistent asthma. Ann Allergy Asthma Immunol 2002 Feb; 88: 227–35
Aubier M, Pieters WR, Schlösser NJJ, et al. Salmeterol/fluticasone propionate (50/500 μg) in combination in a Diskus inhaler (Seretide) is effective and safe in the treatment of steroid-dependent asthma. Respir Med 1999; 93: 876–84
Bateman ED, Britton M, Carrillo J, et al. Salmeterol/fluticasone combination inhaler: a new, effective and well tolerated treatment for asthma. Clin Drug Invest 1998; 16(3): 193–201
Chapman KR, Ringdal N, Backer V, et al. Salmeterol and fluticasone propionate (50/250 μg) administered via combination Diskus inhaler: as effective as when given via separate Diskus inhalers. Can Respir J 1999 Jan–Feb 28; 6(1): 45–51
Van den Berg NJ, Ossip MS, Hederos CA, et al. Salmeterol/fluticasone propionate (50/100 μg) in combination in a Diskus inhaler (Seretide) is effective and safe in children with asthma. Pediatr Pulmonol 2000; 30: 97–105
Ringdal N, Chuchalin A, Chovan L, et al. Evaluation of different inhaled combination therapies (EDICT): a randomised, double-blind comparison of Seretide (50/250 μg bd Diskus vs. formoterol (12 μg bd) and budesonide (800 μg bd) given concurrently (both via Turbuhaler) in patients with moderate-to-severe asthma. Respir Med 2002 Nov; 96: 851–61
Nelson HS, Busse WW, Kerwin E, et al. Fluticasone propionate/salmeterol combination provides more effective asthma control than low-dose inhaled corticosteroid plus montelukast. J Allergy Clin Immunol 2000 Dec; 106(6): 1088–95
Ringdal N, Eliraz A, Pruzinec P, et al. The salmeterol/fluticasone combination is more effective than fluticasone plus oral montelukast in asthma. Respir Med 2003 Mar; 97: 234–41
Malone R, Laforce C, Nimmagadda S, et al. The safety of twice-daily treatment with fluticasone propionate and salmeterol in pediatric patients with persistent asthma. Ann Allergy Asthma Immunol 2005 Jul; 95(1): 66–71
Fitzgerald JM, Boulet L-P, Follows RMA. The CONCEPT trial: a 1-year, multicenter, randomized, double-blind, double-dummy comparison of a stable dosing regimen of salmeterol/fluticasone propionate with an adjustable maintenance dosing regimen of formoterol/budesonide in adults with persistent asthma. Clin Ther 2005 Apr; 27(4): 393–406
Aalbers R, Backer V, Kava TTK, et al. Adjustable maintenance dosing with budesonide/formoterol compared with fixed-dose salmeterol/fluticasone in moderate to severe asthma. Curr Med Res Opin 2004; 20(2): 225–40
Dahl R, Chuchalin A, Lindberg A, et al. EXCEL: regular maintenance therapy with salmeterol/fluticasone propionate combination (SFC) reduces exacerbations more effectively than with formoterol/budesonide combination (FBC) [abstract no. P1977]. Eur Respir J 2004 Sep; 24 Suppl. 48: 309
Bateman ED, Silins V, Bogolubov M. Clinical equivalence of salmeterol/fluticasone propionate in combination (50/100 μg twice daily) when administered via a chlorofluorocarbon-free metered dose inhaler or dry powder inhaler to patients with mild-to-moderate asthma. Respir Med 2001 Feb; 95: 136–46
van Noord JA, Lill H, Carrillo Diaz T, et al. Clinical equivalence of a salmeterol/fluticasone propionate combination product (50/500μg) delivered via a chlorofluorocarbon-free metered-dose inhaler with the Diskus in patients with moderate to severe asthma. Clin Drug Invest 2001; 21(4): 243–55
Bracamonte T, Schauer U, Emeryk A, et al. Efficacy and safety of salmeterol/fluticasone propionate combination delivered by the Diskus or pressurised metered-dose inhaler in children with asthma. Clin Drug Invest 2005; 25(1): 1–11
Davis EA, Bowers B, Pepsin P, et al. The impact of fluticasone propionate/salmeterol combination product compared to oral montelukast on asthma related quality of life [abstract]. Am J Respir Crit Care Med 2001 Apr; 163 (5 Suppl. Pt 2): A506
Edin HM, Lang ML, Vandermeer AK, et al. Fluticasone propionate/salmeterol Diskus combination product improves asthma-related quality of life compared with individual components in asthma patients symptomatic on β2 agonists alone [abstract no. 733]. J Allergy Clin Immunol 2002 Jan; 109 (1 Suppl.): S241
Juniper EF, Jenkins C, Price MJ, et al. Impact of inhaled salmeterol/fluticasone propionate combination product versus budesonide on the health-related quality of life of patients with asthma. Am J Respir Med 2002; 1(6): 435–40
McCarthy TP, Edin HM, House K, et al. The effects of salmeterol/fluticasone combination (SFC) dry powder inhaler (DPI) on asthma control and quality of life, in patients previously treated with inhaled corticosteroids (ICS) [abstract no. P58]. Thorax 2001 Dec; 56 Suppl. 3: iii63-4
Reese PR, Mahajan P, Woodring A. Salmeterol/fluticasone propionate combination product improves quality of life in asthma patients [abstract no. P0325]. Eur Respir J 1998 Sep; 12 Suppl. 28: 35s
Global Initiative for Asthma; National Institutes of Health; National Heart, Lung and Blood Institute. GINA workshop report: global strategy for asthma management and prevention (revised dy2002) [online]. Available from URL: http:// www.ginasthma.com [Accessed 2004 Dec 8]
Nelson HS, Chapman KR, Pyke SD, et al. Enhanced synergy between fluticasone propionate and salmeterol inhaled from a single inhaler versus separate inhalers. J Allergy Clin Immunol 2003 Jul; 112(1): 29–36
Lyseng-Williamson KA, Plosker GL. Inhaled salmeterol/fluticasone propionate combination: a pharmacoeconomic review of its use in the management of asthma. Pharmacoeconomics 2003; 21(13): 951–89
Johansson G, Price MJ, Sondhi S. Cost-effectiveness analysis of salmeterol/fluticasone propionate 50/100μg vs fluticasone propionate 100μg in adults and adolescents with asthma III: results. Pharmacoeconomics 1999; 16 Suppl. 2: 15–21
Lundbäck B, Jenkins C, Price MJ, et al. Cost-effectiveness of salmeterol/fluticasone propionate combination product 50/250 μg twice daily and budesonide 800 μg twice daily in the treatment of adults and adolescents with asthma. Respir Med 2000; 94: 724–32
O’Connor RD, Nelson H, Borker R, et al. Cost effectiveness of fluticasone propionate plus salmeterol versus fluticasone propionate plus montelukast in the treatment of persistent asthma. Pharmacoeconomics 2004; 22(12): 815–25
Palmqvist M, Price MJ, Sondhi S. Cost-effectiveness analysis of salmeterol/fluticasone propionate 50/250μg vs fluticasone propionate 250μg in adults and adolescents with asthma IV: results. Pharmacoeconomics 1999; 16 Suppl. 2: 23–8
Pieters WR, Lundbäck B, Sondhi S, et al. Cost-effectiveness analysis of salmeterol/fluticasone propionate 50/500μg vs fluticasone propionate 500μg in patients with corticosteroid-dependent asthma V: results. Pharmacoeconomics 1999; 16 Suppl. 2: 29–34
Sheth K, Borker R, Emmett A, et al. Cost-effectiveness comparison of salmeterol/fluticasone propionate versus montelukast in the treatment of adults with persistent asthma. Pharmacoeconomics 2002; 20(13): 909–18
Price MJ, Briggs AH. Development of an economic model to assess the cost effectiveness of asthma management strategies. Pharmacoeconomics 2002; 20(3): 183–94
Pieters WR, Wilson KK, Smith HCE, et al. Salmeterol/fluticasone propionate versus fluticasone propionate plus montelukast: a cost-effective comparison for asthma. Treat Respir Med 2005; 4(2): 129–38
British Thoracic Society, Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Thorax 2003 Feb; 58 Suppl. 1: 1–94
Rabe KF, Adachi M, Lai CKW, et al. Worldwide severity and control of asthma in children and adults: the global asthma insights and reality surveys. J Allergy Clin Immunol 2004 Jul; 114(1): 40–7
Bateman ED, Bousquet J, Braunstein GL. Is overall asthma control being acheived? A hypothesis-generating study. Eur Respir J 2001; 17: 589–95
Stoloff SW, Stempel DA, Meyer J, et al. Improved refill persistence with fluticasone propionate and salmeterol in a single inhaler compared with other controller therapies. J Allergy Clin Immunol 2004 Feb; 113(2): 245–51
Author information
Authors and Affiliations
Additional information
Various sections of the manuscript reviewed by: W. Boonsawat, Department of Medicine, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand; P.W. Ind, Clinical Investigation Unit, Respiratory Medicine, Hammersmith Hospital, London, UK; G. Johansson, Nyby Health Care Centre, Uppsala, Sweden; W.R. Pieters, Elkerliek Ziekenhuis, Helmond, The Netherlands; A.J. Tatum, Asthma Inc. Clinical Research Center, Seattle, Washington, USA.
Data Selection
Sources: Medical literature published in any language since 1980 on salmeterol/fluticasone propionate, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE and EMBASE search terms were ‘salmeterol fluticasone propionate’ and ‘asthma’. AdisBase search terms were ‘salmeterol/fluticasone propionate’ and ‘asthma’. Searches were last updated 12 July 2005.
Selection: Studies in patients with asthma who received salmeterol/fluticasone propionate. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Asthma, fluticasone propionate, salmeterol, salmeterol/fluticasone propionate, pharmacodynamics, pharmacoeconomics, pharmacokinetics, therapeutic use.
Rights and permissions
About this article
Cite this article
Reynolds, N.A., Lyseng-Williamson, K.A. & Wiseman, L.R. Inhaled Salmeterol/Fluticasone Propionate. Drugs 65, 1715–1734 (2005). https://doi.org/10.2165/00003495-200565120-00012
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200565120-00012