Summary
Abstract
Insulin aspart (NovoRapid®, NovoLog®) is a short-acting insulin analogue, which has a faster onset and shorter duration of action than regular human insulin.
Insulin aspart administered immediately before meals provided significantly greater improvements in glycosylated haemoglobin and better postprandial glycaemic control than regular human insulin administered 30 minutes before meals, when used in a basal-bolus regimen with neutral protamine Hagedorn (NPH) insulin, in randomised, nonblind studies in patients with type 1 diabetes mellitus.
In patients with type 2 diabetes, insulin aspart provided similar glycaemic control to regular human insulin, administered in a basal-bolus regimen with NPH insulin. Small studies suggest that the use of insulin aspart in combination with oral hypoglycaemic agents may be beneficial. Insulin aspart, administered by continuous subcutaneous insulin infusion (CSII) provided better glycaemic control than insulin aspart multiple daily injection regimens in patients with type 1 (but not type 2) diabetes, and had similar efficacy to CSII with insulin lispro or regular human insulin in type 1 diabetes. Limited studies show insulin aspart to be effective in children, adolescents and young adults with type 1 diabetes.
Insulin aspart had a tolerability profile similar to that of regular human insulin in clinical trials. The incidence of major or nocturnal hypoglycaemic events reported in patients receiving insulin aspart was lower than that of regular human insulin in several studies.
In conclusion, insulin aspart, administered immediately before meals in a basal-bolus regimen with NPH insulin, provided better long-term glycaemic control than regular human insulin administered 30 minutes before meals in patients with type 1 diabetes, and was as effective as regular human insulin in patients with type 2 diabetes. A significantly lower risk of hypoglycaemia was seen in several trials. Insulin aspart CSII provided better glycaemic control than insulin aspart multiple daily subcutaneous injection (MDI) in patients with type 1 (but not type 2) diabetes and had similar efficacy to CSII with insulin lispro or regular human insulin in type 1 diabetes. Insulin aspart is an effective and well tolerated alternative to regular human insulin and insulin lispro for the maintenance of glycaemic control in patients with type 1 or 2 diabetes.
Pharmacodynamic Profile
The substitution of one amino acid in human insulin to make insulin aspart, a short-acting insulin analogue, facilitates absorption following subcutaneous administration.
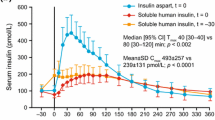
Glycaemic control (24-hour) with insulin aspart, administered immediately before meals, was at least as good as that with human insulin and similar to that with insulin lispro in clinical studies involving adult patients with type 1 (and some with type 2) diabetes. The duration of action of insulin aspart is shorter than that of human insulin. However, administration of insulin aspart immediately before or 15 minutes after a meal appears not to affect glycaemic control.
Postprandial glycaemic control was more effective with insulin aspart than with regular human insulin in patients with type 1 diabetes, but there were no differences between these treatments in patients with type 2 diabetes. Postprandial glycaemic control was similar in patients with type 1 diabetes receiving insulin aspart, insulin lispro or regular human insulin by CSII. In patients with type 2 diabetes, similar improvements were seen with multiple-dose or CSII insulin aspart administration.
Pharmacokinetic Profile
Insulin aspart exhibits linear pharmacokinetics. Subcutaneous insulin aspart is absorbed faster than human insulin, although bioavailability is similar. Insulin aspart is eliminated more quickly from the body than human insulin, but total plasma clearance is the same. The pharmacokinetic parameters of insulin aspart and insulin lispro are similar.
In healthy volunteers, insulin aspart was associated with significantly lower interindividual variability in several pharmacokinetic properties. Insulin aspart was absorbed faster than regular human insulin following subcutaneous administration in children and adolescents. Hepatic or renal impairment does not appear to alter insulin aspart pharmacokinetics.
Therapeutic Efficacy
Insulin aspart, administered immediately before meals, provided small but significantly greater improvements in glycosylated haemoglobin (HbA1c) than regular human insulin, administered 30 minutes before meals, when used with NPH insulin in a basal-bolus regimen in three studies of 12–52 weeks’ duration in patients with type 1 diabetes; equal efficacy was seen in a 64-week trial. In an extension to one trial, insulin aspart maintained superiority over regular human insulin at 2.5 years. Glycaemic control was more effective in patients receiving insulin detemir plus insulin aspart than in those receiving NPH insulin plus regular human insulin and as effective as NPH insulin plus insulin aspart in 16-to 18-week studies. In two large, 12-or 26-week trials in patients with type 2 diabetes, insulin aspart showed similar glycaemic control to regular human insulin administered with NPH insulin in a basal-bolus regimen.
Insulin aspart, insulin lispro and regular human insulin provided similar glycaemic control at 16 weeks in patients with type 1 diabetes when administered via CSII. Insulin aspart CSII provided better glycaemic control than insulin aspart MDI regimens in patients with type 1 diabetes and similar efficacy in patients with type 2 diabetes.
In a 12-week study in patients aged 7–17 years, insulin aspart provided similar glycaemic control to regular human insulin when administered in a basal-bolus regimen with NPH insulin. Insulin aspart CSII provided better glycaemic control than once-daily insulin glargine in patients aged 8–21 years with type 1 diabetes.
Tolerability
Insulin aspart had a tolerability profile similar to that of regular human insulin in patients with diabetes. The incidence of hypoglycaemic events reported in patients receiving insulin aspart was generally similar to that of regular human insulin. However, insulin aspart had a significantly lower incidence of nocturnal hypoglycaemic events in several studies and of major hypoglycaemic episodes in one trial. The incidence of other adverse events and immunogenic response did not differ from that seen with regular human insulin.





Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
World Health Organisation. Diabetes (WHO global strategy on diet, physical activity and health) [online]. Available from URL: http://www.who.int/dietphysicalactivity/publications/facts/diabetes/en/ [Accessed 2004 Jun 8]
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993 Sep 30; 329(14): 977–86
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998 Sep 12; 352: 837–53
Stratton IM, Adler AI, Neil HAW, et al., on behalf of the UK Prospective Diabetes Study Group. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000 Aug 12; 321: 405–12
American Diabetes Association. 2004 Clinical Practice Recommendations. Diabetes Care 2004 Jan; 27 Suppl. 1: S1–143
Gummerson I. Insulin analogues revisited. Hosp Pharmacist 2003 Apr; 10(4): 165–7, 169–73
Haycox A. Insulin aspart: a comprehensive evidence-based medicine review. Clin Drug Invest. In press
Gammeltoft S, Hansen BF, Dideriksen L, et al. Insulin aspart: a novel rapid-acting human insulin analogue. Expert Opin Invest Drugs 1999; 8(9): 1431–41
Pettitt DJ, Ospina P, Kolaczynski JW, et al. Comparison of an insulin analog, insulin aspart, and regular human insulin with no insulin in gestational diabetes mellitus. Diabetes Care 2003 Jan; 26(1): 183–6
Umpierrez GE, Cuervo R, Karabell A, et al. Treatment of diabetic ketoacidosis with subcutaneous insulin aspart. Diabetes Care 2004; 28(8): 1873–8
Chapman TM, Noble S, Goa KL. Insulin aspart: a review of its use in the management of type 1 and 2 diabetes mellitus. Drugs 2002; 62(13): 1945–81
Kaku K, Matsuda M, Urae A, et al. Pharmacokinetics and pharmacodynamics of insulin aspart, a rapid-acting analog of human insulin, in healthy Japanese volunteers. Diabetes Res Clin Pract 2000 Aug; 49(2–3): 119–26
Nosek L, Heinemann L, Kaiser M, et al. No increase in the duration of action with rising doses of insulin aspart [abstract no. 551-P]. Diabetes 2003 Jun; 52 Suppl. 1: A128–9
Heinemann L, Weyer C, Rauhaus M, et al. Variability of the metabolic effect of soluble insulin and the rapid-acting insulin analog insulin aspart. Diabetes Care 1998 Nov; 21(11): 1910–4
Perriello G, Avogaro A, Emanuele B, et al. Superior meal-time glucose control with insulin aspart (NovoLog®) compared with human insulin in both normal-weight and overweight people with type 2 diabetes: a randomized, stratified, double-blind, crossover trial [abstract no. 452-P]. Diabetes 2002 Jun; 51 Suppl. 2: A111
Lindholm A, McEwen J, Riis AP. Improved postprandial glycemic control with insulin aspart: a randomized double-blind cross-over trial in type 1 diabetes. Diabetes Care 1999 May; 22(5): 801–5
Rosenfalck AM, Thorsby P, Kjems L, et al. Improved postprandial glycaemic control with insulin aspart in type 2 diabetic patients treated with insulin. Acta Diabetol 2000 Mar; 37(1): 41–6
Home PD, Lindholm A, Hylleberg B, et al., for the U.K. Insulin Aspart Study Group. Improved glycemic control with insulin aspart: a multicenter randomized double-blind crossover trial in type 1 diabetic patients. Diabetes Care 1998 Nov; 21(11): 1904–9
Plank J, Wutte A, Brunner G, et al. A direct comparison of insulin aspart and insulin lispro in patients with type 1 diabetes. Diabetes Care 2002 Nov; 25(11): 2053–7
Homko C, Deluzio A, Jimenez C, et al. Comparison of insulin aspart and lispro: pharmacokinetic and metabolic effects. Diabetes Care 2003 Jul; 26(7): 2027–31
DeVries JH, Lindholm A, Jacobsen JL, et al., on behalf of the Tri-Continental Insulin Aspart Study Group. A randomized trial of insulin aspart with intensified basal NPH insulin supplementation in people with type 1 diabetes. Diabet Med 2003 Apr; 20(4): 312–8
Home PD, Lindholm A, Riis A, for the European Insulin Aspart Study Group. Insulin aspart vs. human insulin in the management of long-term blood glucose control in type 1 diabetes mellitus: a randomized controlled trial. Diabet Med 2000 Nov; 17(11): 762–70
Raskin P, Guthrie RA, Leiter L, et al. Use of insulin aspart, a fast-acting insulin analog, as the mealtime insulin in the management of patients with type 1 diabetes. Diabetes Care 2000 May; 23(5): 583–8
Tamás G, Marre M, Astorga R, et al. Glycaemic control in type 1 diabetic patients using optimised insulin aspart or human insulin in a randomised multinational study. Diabetes Res Clin Pract 2001 Nov; 54(2): 105–14
Home P, Bartley P, Russell-Jones D, et al. Insulin detemir offers improved glycemic control compared with NPH insulin in people with type 1 diabetes: a randomized clinical trial. Diabetes Care 2004 May; 27(5): 1081–7
Hermansen K, Fontaine P, Kukolja KK, et al. Insulin analogues (insulin detemir and insulin aspart) versus traditional human insulins (NPH insulin and regular human insulin) in basal-bolus therapy for patients with type 1 diabetes. Diabetologia 2004 Mar 12; 47: 622–9
Bretzel RG, Arnolds S, Medding J, et al. A direct efficacy and safety comparison of insulin aspart, human soluble insulin, and human premix insulin (70/30) in patients with type 2 diabetes. Diabetes Care 2004 May; 27(5): 1023–7
McGill J, Raskin P, Kilo C, et al. Human insulin analog, insulin aspart, is a mealtime insulin comparable to human insulin in type 2 diabetes [abstract no. 894]. Diabetologia 1999 Aug; 42 Suppl. 1: A238
Bode B, Weinstein R, Bell D, et al. Comparison of insulin aspart with buffered regular insulin and insulin lispro in continuous subcutaneous insulin infusion: a randomized study in type 1 diabetes. Diabetes Care 2002 Mar; 25(3): 439–44
DeVries JH, Snoek FJ, Kostense PJ, et al. A randomized trial of continuous subcutaneous insulin infusion and intensive injection therapy in type 1 diabetes for patients with long-standing poor glycaemic control. Diabetes Care 2002 Nov; 25(11): 2074–80
Raskin P, Bode BW, Marks JB, et al. Continuous subcutaneous insulin infusion and multiple daily injection therapy are equally effective in type 2 diabetes: a randomized, parallel-group, 24-week study. Diabetes Care 2003 Sep; 26(9): 2598–603
Brunner GA, Hirschberger S, Sendlhofer G, et al. Post-prandial administration of the insulin analogue insulin aspart in patients with type 1 diabetes mellitus. Diabet Med 2000 May; 17(5): 371–5
Mortensen HB, Lindholm A, Olsen BS, et al. Rapid appearance and onset of action of insulin aspart in paediatric subjects with type 1 diabetes. Eur J Pediatr 2000 Jul; 159(7): 483–8
Doyle EA, Weinzimer SA, Steffen AT, et al. A randomized, prospective trial comparing the efficacy of continuous subcutaneous insulin infusion with multiple daily injections using insulin glargine. Diabetes Care 2004 Jul; 27(7): 1554–8
Danne T, Aman J, Schober E, et al. A comparison of postprandial and preprandial administration of insulin aspart in children and adolescents with type 1 diabetes. Diabetes Care 2003 Aug; 26(8): 2359–64
Krones R, Heise T, Basir S, et al. Time-action profiles of insulin aspart and human regular insulin in elderly and middle-aged subjects with type 2 diabetes [abstract no. 560-P]. Diabetes 2004 Jun; 53 Suppl. 2: A133
Gallagher A, Home PD. The effect of insulin aspart on postprandial metabolism and lipid profile in type 2 diabetes [abstract no. 788]. Diabetologia 2002 Aug; 45 Suppl. 2: A253–4
Hamilton-Wessler M, Buchanan T, Hathout E, et al. Time-action profile for intravenously infused insulin aspart and insulin lispro in type 1 diabetic subjects [abstract no. 403-P]. Diabetes 2002 Jun; 51 Suppl. 2: A99
Novo Nordisk Limited. NovoRapid: summary of product characteristics [online]. Available from URL: http://emc.medicines.org.uk [Accessed 2004 Apr 27]
Novo Nordisk Pharmaceuticals Inc.. NovoLog®: insulin aspart (rDNA origin) injection. Prescribing information [online]. Available from URL: http://www.novolog.com [Accessed 2004 May 5]
Lindholm A, Sasaki T, Edwards A, et al. Dose dependency in absorption of insulin aspart in healthy Caucasians and Japanese [abstract no. 1840-PO]. Diabetes 2001 Jun; 50 Suppl. 2: A441
Lindholm A, Jacobsen LV. Clinical pharmacokinetics and pharmacodynamics of insulin aspart. Clin Pharmacokinet 2001; 40(9): 641–59
von Mach M-A, Brinkmann C, Hansen T, et al. Differences in pharmacokinetics and pharmacodynamics of insulin lispro and aspart in healthy volunteers. Exp Clin Endocrinol Diabetes 2002 Nov; 110(8): 416–9
Lindström T, Hedman CA, Arnqvist HJ. Use of a novel double-antibody technique to describe the pharmacokinetics of rapid-acting insulin analogs. Diabetes Care 2002 Jun; 25(6): 1049–54
Plum A, Larsen PS, Larsen UD, et al. Determination of in vitro plasma protein binding of insulin aspart and insulin detemir by equilibrium dialysis [abstract no. 886]. Diabetologica 1999; 42 Suppl. 1: A236
Home PD, Barriocanal L, Lindholm A. Comparative pharmacokinetics and pharmacodynamics of the novel rapid-acting insulin analogue, insulin aspart, in healthy volunteers. Eur J Clin Pharmacol 1999 May; 55(3): 199–203
Robinson RTCE, Harris ND, Ireland RH, et al. Comparative effect of human soluble insulin and insulin aspart upon hypoglycaemia-induced alterations in cardiac repolarization. Br J Clin Pharmacol 2003 Mar; 55(3): 246–51
Hedman CA, Lindstrom T, Arnqvist HJ. Direct comparison of insulin lispro and aspart shows small differences in plasma insulin profiles after subcutaneous injection in type 1 diabetes [letter]. Diabetes Care 2001 Jun; 24(6): 1120–1
Lyness W, Tyler J, Lawrence A. Pharmacokinetics of the rapid-acting insulin-analog, insulin aspart, is independent of hepatic function [abstract no. 1843-PO]. Diabetes 2001 Jun; 50 Suppl. 2: A442
Lyness W, Tyler JF, Lawrence A. Renal impairment does not affect insulin aspart pharmacokinetics in type 1 diabetes [abstract no. 1842-PO]. Diabetes 2001 Jun; 50 Suppl. 2: A441-2
Lyness W, Tyler J, Lawrence A. BMI does not affect insulin aspart pharmacokinetics in type I diabetes [abstract no. 499-P]. Diabetes 2001 Jun; 50 Suppl. 2: A124
Tsalikian E. Insulin aspart in pediatric and adolescent patients with type 1 diabetes [abstract no. 767]. Diabetologia 2000 Aug; 43 Suppl. 1: 200
Poulsen MK, Henriksen JE, Hother-Nielsen O, et al. The combined effect of triple therapy with rosiglitazone, metformin, and insulin aspart in type 2 diabetic patients. Diabetes Care 2003 Dec; 26(12): 3273–9
de Boer H., Jansen M, Koerts J, et al. Prevention of weight gain in type 2 diabetes requiring insulin treatment. Diabetes Obes Metab 2004 Mar; 6(2): 114–9
Amiel S, Home PD, Jacobsen JL, et al. Insulin aspart safe for long-term treatment [abstract no. 802]. Diabetologia 2001; 44 Suppl. 1: A209
Bott U, Ebrahim S, Hirschberger S, et al. Effect of the rapid-acting insulin analogue insulin aspart on quality of life and treatment satisfaction in patients with type 1 diabetes. Diabet Med 2003 Aug; 20(8): 626–34
Heller SR, Colagiuri S, Vaaler S, et al. Hypoglycaemia with insulin aspart: a double-blind, randomised, crossover trial in subjects with type 1 diabetes. Diabet Med 2004 Jul; 21(7): 769–75
Lindholm A, Jensen LB, Home PD, et al. Immune responses to insulin aspart and biphasic insulin aspart in people with type 1 and type 2 diabetes. Diabetes Care 2002 May; 25(5): 876–82
European Diabetes Policy Group 1999. A desktop guide to type 2 diabetes mellitus. Diabet Med 1999; 16: 716–30
Gerich JE. Novel insulins: expanding options in diabetes management. Am J Med 2002 Sep; 113(4): 308–16
Lindholm A. New insulins in the treatment of diabetes mellitus. Best Pract Res Clin Gastroenterol 2002 Jun; 16(3): 475–92
Chan JL, Abrahamson MJ. Pharmacological management of type 2 diabetes mellitus: rationale for rational use of insulin. Mayo Clin Proc 2003; 78(4): 459–67
McIntosh A, Hutchinson A, Home PD, et al. Clinical guidelines and evidence review for type 2 diabetes: management of blood glucose [online]. Available from URL: http://www.shef.ac.uk/guidelines/ [Accessed 2004 Jun 16]
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: B. Bode, Atlanta Diabetes Associates, Atlanta, Georgia, USA; B.O. Boehm, Abteilung Innere Medizin I, Universitätsklinikum Ulm, Ulm, Germany; J.-W. Chen, Department of Endocrinology and Diabetes, Aarhus University Hospital, Aarhus, Denmark; J.S. Christiansen, Department of Endocrinology and Diabetes, Aarhus University Hospital, Aarhus, Denmark; J.A. Davidson, Endocrine & Diabetes Associates of Texas, Dallas, Texas, USA; J.H. DeVries, Department of Internal Medicine, Academic Medical Center, Amsterdam, The Netherlands; S. Heller, Clinical Sciences Centre, University of Sheffield, Sheffield, United Kingdom; I. Raz, Diabetes Center, Hadassah Ein-Kerem University Hospital, Jerusalem, Israel; G. Tamás, First Department of Medicine, Diabetes Unit, National Centre for Diabetes Care, Budapest, Hungary.
Data Selection
Sources: Medical literature published in any language since 1980 on insulin aspart, identified using Medline and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: Medline search terms were ‘insulin aspart’ or ‘ASP-B28’. EMBASE search terms were ‘insulin aspart’. AdisBase search terms were ‘insulin aspart’ or ‘ASP-B28’. Searches were last updated 5 July 2004.
Selection: Studies in patients with type 1 or 2 diabetes mellitus who received insulin aspart. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Insulin aspart, diabetes mellitus, insulin analogue, pharmacodynamics, pharmacokinetics, therapeutic use.
Rights and permissions
About this article
Cite this article
Reynolds, N.A., Wagstaff, A.J. Insulin Aspart. Drugs 64, 1957–1974 (2004). https://doi.org/10.2165/00003495-200464170-00013
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200464170-00013