Abstract
-
▴ Zoledronic acid (zoledronate) is a new generation bisphosphonate that inhibits osteoclast bone resorption. It was much more potent than other bisphosphonates at inhibiting 1,25-dihydroxyvitamin D3-induced hypercalcaemia in a rat model and calcium release in vitro.
-
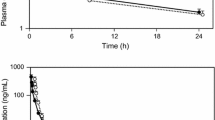
▴ A single 5-minute intravenous infusion of zoledronic acid (4 or 8mg) was significantly more effective than a 2-hour infusion of pamidronic acid (pamidronic acid disodium, pamidronate disodium) [90mg] in normalising serum calcium levels in patients with hypercalcaemia of malignancy and resulted in a significantly longer median time to relapse (pooled analysis from 2 randomised, double-blind, parallel-group trials).
-
▴ There were no differences in tolerability between zoledronic acid and pamidronic acid in comparative trials; the most common events in pivotal trials were fever, anaemia, nausea, constipation and dyspnoea. Fever, hypophosphataemia and hypocalcaemia were the most common events in a small phase I trial.



Similar content being viewed by others
References
Green MD. Oral bisphosphonates and malignancy. Med J Aust 1997 Aug 18; 167:211–2
Body JJ, Coleman RE, Piccart M. Use of bisphosphonates in cancer patients. Cancer Treat Rev 1996; 22(4): 265–87
Body JJ. Clinical research update: zoledronate. Cancer 1997 Oct 15; 80 Suppl.: 1699–701
Novartis Pharma. Zometa International Monograph. Novartis Pharma (Data on file)
Berenson JR, Vescio R, Henick K, et al. A phase I, open label, dose ranging trial of intravenous bolus zoledronic acid, a novel bisphosphonate, in cancer patients with metastatic bone disease. Cancer 2001 Jan 1; 91(1): 144–54
Green JR, Müller K, Jaeggi KA. Preclinical pharmacology of CGP 42’446, a new, potent, heterocyclic bisphosphonate compound. J Bone Miner Res 1994 May; 9: 745–51
Pataki A, Müller K, Green JR, et al. Effects of short-term treatment with the bisphosphonates zoledronate and pamidronate on rat bone: a comparative histomorphometric study on the cancellous bone formed before, during, and after treatment. Anat Rec 1997 Dec; 249: 458–68
Evans CE, Braidman IP. Effects of two novel bisphosphonates on bone cells in vitro. Bone Miner 1994 Aug; 26: 95–107
Green JR, Seltenmeyer Y, Jaeggi KA, et al. Renal tolerability profile of novel, potent bisphosphonates in two short-term rat models. Pharmacol Toxicol 1997 May; 80: 225–30
Novartis Pharma. Zometa: product information [in German]. Novartis Pharma
Berenson J, Ravera C, Ma P, et al. Population pharmacokinetics (PK) of Zometa [abstract no. 814]. Proceedings of the American Society of Clinical Oncology; 2000 May 20–23; New Orleans, 209a
Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol 2001 Jan 15; 19(2): 558–67
Body JJ, Lortholary A, Romieu G, et al. A dose-finding study of zoledronate in hypercalcemic cancer patients. J Bone Miner Res 1999; 14(9): 1557–61
Novartis alters zometa study design due to renal function concerns. The Pink Sheet, 2000 Jun 26; 21
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheer, S.M., Noble, S. Zoledronic Acid. Drugs 61, 799–805 (2001). https://doi.org/10.2165/00003495-200161060-00010
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200161060-00010