Summary
-
▴ Zanamivir is the first of a new class of selective influenza virus neuraminidase inhibitors. It inhibits both influenza A and influenza B virus replication in vitro.
-
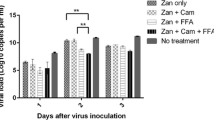
▴ In the ferret model of influenza, zanamivir reduced viral replication and diminished pyrexia associated with the infection.
-
▴ Repeated passageof influenza virus in the presence of zanamivir could produce resistance in vitro. However, there have been no changes in sensitivity to zanamivir in any influenza virus isolates from patients receiving zanamivir in clinical trials.
-
▴ In experimental infection in humans, in which the virus replicates only in the nasal passages, intranasal zanamivir (3.6 to 16mg) prevented infection with influenza virus. A combination of inhaled (10mg) and intranasal (6.4mg) zanamivir for 14 days was effective in preventing influenza in a nursing home setting during an influenza A virus outbreak.
-
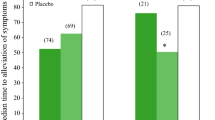
▴ Inhaled zanamivir (10mg) with or without intranasal zanamivir (6.4mg) reduced the time to alleviation of influenza symptoms compared with placebo in patients with confirmed infection.
Similar content being viewed by others
References
Madren LK, Shipman JC, Hayden FG. In vitro inhibitory effects of combinations of anti-influenza agents. Antiviral Chem Chemother 1995 Mar; 6: 109–13
Guay DRP. Amantadine and rimantadine prophylaxis of influenza A in nursing homes: a tolerability perspective. Drugs Aging 1994 Jul; 5: 8–19
Whittington A, Bethell R. Recent developments in the antiviral therapy of influenza. Expert Opinion on Therapeutic Patents 1995 Aug; 5: 793–803
Woods JM, Bethell RC, Coates JA, et al. 4-Guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid is a highly effective inhibitor both of the sialidase (neuraminidase) and of growth of a wide range of influenza A and B viruses in vitro. Antimicrob Agents Chemother 1993 Jul; 37: 1473–9
Bethell RC, Smith PW. Sialidase as a target for inhibitors of influenza virus replication. Expert Opin Invest Drug 1997 Oct; 6: 1501–9
Hayden FG, Rollins BS, Madren LK. Anti-influenza virus activity of the neuraminidase inhibitor 4-guanidino-Neu5-Ac2en in cell culture and in human respiratory epithelium. Antiviral Res 1994 Oct; 25: 123–31
Thomas GP, Forsyth M, Penn CR, et al. Inhibition of the growth of influenza viruses in vitro by 4-guanidino-2,4-dideoxy-N-acetylneuraminic acid. Antiviral Res 1994 Aug; 24: 351–6
Ryan DM, Ticehurst J, Dempsey MH, et al. Inhibition of influenza virus replication in mice by GG167 (4-guanidino-2,4-dideoxy-2,3,-dehydro-N-acetylneuraminic acid) is consistent with extracellular activity of viral neuraminidase (sialidase). Antimicrob Agents Chemother 1994 Oct; 38: 2270–5
Ryan DM, Ticehurst J, Dempsey MH. GG167 (4-guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid) is a potent inhibitor of influenza virus in ferrets. Antimicrob Agents Chemother 1995 Nov; 39: 2583–4
McKimm-Breschkin JL, Blick TJ, Sahasrabudhe A, et al. Generation and characterization of variants of NWS/G70C influenza virus after in vitro passage in 4-amino-neu5Ac2en and 4-guanidino-neu5Ac2en. Antimicrob Agents Chemother 1996 Jan; 40: 40–6
Colacino JM, Laver WG, Air GM. Selection of influenza A and B viruses for resistance to 4-guanidino-Neu5Ac2en in cell culture. J Infect Dis 1997 Aug; 176 Suppl. 1: S66–8
Gubareva LV, Robinson MJ, Bethell RC, et al. Catalytic and framework mutations in the neuraminidase active site of influenza viruses that are resistant to 4-guanidino-Neu5Ac2en. J Virol 1997 May; 71: 3385–90
Barnett J, Dempsey M, Tisdale M, et al. Susceptibility monitoring of influenza virus clinical isolates to the neuraminidase inhibitor zanamivir ((GG167) during phase II clinical efficacy trials[abstract no. H-93]. 37th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1997 Sep 28–Oct 1; Toronto, 280
Efthymiopoulos C, Barrington P, Patel J, et al. Pharmacokinetics of the neuraminidase inhibitor 4-guanidino Neu5Ac2en (GG167) following intravenous, intranasal and inhaled administration in man [abstract]. 34th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1994 Oct 4–7; Orlando (FL), 265
Newman SP, Brown J, Pickford M, et al. Deposition pattern in the respiratory tract of the neuraminidase inhibitor zanamivir; a gamma scintigraphic study [abstract no. H-134]. 37th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1997 Sep 28–Oct 1; Toronto, 237
Hussey E, Hayden F, Grosse C, et al. Serum and urine GG167 concentrations in healthy subjects inoculated experimentally with influenza A virus [abstract]. 35th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1995 Sep 17–20; San Francisco, 25
Peterson MC, Sweeney KR, Hsyu PH. Renal disposition and drug screening of GR121167 (GG167) in the isolated perfused rat kidney [abstract]. Pharm Res 1996 Sep; 13 Suppl.: 448
Schilling M, Povinelli L, Krause P, et al. Efficacy of zanamivir for chemoprophylaxis of nursing home influenza A outbreaks [abstract no. H-92]. 37th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1997 Sep 28–Oct 1; Toronto, 230
Hayden FG, Treanor JJ, Betts RF, et al. Safety and efficacy of the neuraminidase inhibitor GG167 in experimental human influenza. JAMA 1996 Jan 24–31; 275: 295–9
Hayden FG, Osterhaus ADME, Treanor JJ, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. N Engl JMed 1997 Sep 25; 337: 874–80
Aoki F, Fleming D, Lacey L, et al. Impact of treatment of influenza with zanamivir on patients’ health status, sleep quality, productivity and healthcare use [abstract no. N-15]. 37th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1997 Sep 28–Oct 1; Toronto, 384
Rodriguez WJ, Hall CB, Welliver R. et al. Efficacy and safety of aerosolized ribavirin in young children hospitalized with influenza: a double-blind, multicenter, placebo-controlled trial. J Pediatr 1994; 125: 129–135
Cass LMR. Efthymiopoulos C, Bye A. An overview of the pharmacokinetics of the neuraminidase inhibitor GG167 [abstract]. Options for the control of influenza III. 1996 May; Cairns
Data on file. Glazo Wellcome
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Waghorn, S.L., Goa, K.L. Zanamivir. Drugs 55, 721–725 (1998). https://doi.org/10.2165/00003495-199855050-00015
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199855050-00015