Summary
Synopsis
Recombinant granulocyte-macrophage colony-stimulating factor (rGM-CSF) is a polypeptide hormone produced through recombinant DNA technologies in glycosylated (yeast or mammalian expression systems) or nonglycosylated (Escherichia coli expression system) form. It is a multilineage haematopoietin which stimulates proliferation and differentiation of bone marrow myeloid progenitors and increases peripheral white blood cell counts when administered systemically. Treatment is generally well tolerated, although mild to moderate flu-like symptoms are common and rGM-CSF-induced fever and fluid retention may be problematic in occasional patients.
rGM-CSF accelerates recovery of peripheral neutrophil counts after bone marrow transplantation, and results of a placebo-controlled randomised trial correlate this with reduced infectious episodes and shortened length of hospitalisation in patients with lymphoid malignancies. A substantial number of patients with graft failure after bone marrow transplantation also respond to rGM-CSF. The duration of myelosuppression secondary to cancer chemotherapy can be significantly reduced by rGM-CSF which has permitted investigation of antineoplastic dose-intensity escalation.
In some haematopoietic disorders (e.g. aplastic anaemia, myelodysplasia and neutropenia secondary to HIV infection and antiviral therapy), rGM-CSF produces clinically useful increases in peripheral blood granulocyte counts, although the effect is generally not sustained after drug withdrawal. The potential for rGM-CSF to stimulate proliferation of the abnormal clone in myelodysplasia and in acute myelogenous leukaemia following induction therapy is of concern. Available data suggest, however, that with appropriate monitoring and exclusion of high-risk patients this serious potential risk can be avoided, and that myelopoiesis is enhanced in such patients by rGM-CSF treatment.
Recombinant colony-stimulating factors are a new therapeutic modality; hence many aspects of their use remain to be clarified. Nonetheless, as one of a small group of novel agents rGM-CSF has major potential in the management of myelosuppression secondary to cytoreductive therapy with or without bone marrow transplantation, and in amelioration of disturbed myelopoiesis. It represents an important application of biotechnology to a difficult area of therapeutics.
Pharmacological Properties
Endogenous GM-CSF is produced by T-lymphocytes, macrophages, fibroblasts and endothelial cells, and participates both in the complex regulation of blood cell formation and in activation of mature leucocytes. It is a polypeptide which is variably glycosylated in its native state although the carbohydrate content is not essential for its biological effects, and the 3 available recombinant forms (which differ in extent of glycosylation) are similarly active in vivo. Proliferative activity and priming of mature cells are manifest at similar picomolar concentrations of GM-CSF, and it is the programming of the cell which appears to determine the response to binding of GM-CSF to its cell surface receptor.
In concert with other colony-stimulating factors, GM-CSF facilitates lineage commitment and subsequently supports or amplifies the clonogenic activity of lineage-restricted factors, with the strongest effect seen on the granulocyte-macrophage lineage.
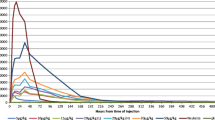
A biphasic response was seen when rGM-CSF was administered in doses up to 1000 µg/m2/day or 60 µg/kg/day by subcutaneous or intravenous routes in phase I/II trials. Peripheral blood leucocyte counts decreased rapidly and profoundly secondary to sequestration within the lungs. Re-entry of these cells into the circulation restores counts to baseline in 2 to 4 hours and thereafter an increase in the proliferative fraction of haematopoietic cells in bone marrow probably accounts for the progressive rise in the number of neutrophils, eosinophils and monocytes. This effect is dose-proportional.
GM-CSF stimulates proliferation of leukaemic progenitors from patients with acute myeloid leukaemia without stimulating differentiation. In contrast, the abnormal clone from myelodysplastic patients can be induced with GM-CSF to differentiate in vitro although karyotype anomalies persist. In vitro studies suggest that stimulation of nonhaematological cancer cells at physiological concentrations of GM-CSF is unlikely.
The priming effects of GM-CSF on mature leucocytes which include increased expression of other cytokines, secretion of granule contents, augmentation of antigen presentation, altered chemotaxis, and enhanced phagocytosis, oxidative metabolism and antibody dependent cell-mediated cytotoxicity probably serve to increase the host response to infection. Administration of murine rGM-CSF to mice injected with lethal inocula of, for example, Pseudomonas aeruginosa improved their survival relative to controls. There are several reports of refractory infection in seriously ill neutropenic patients resolving after addition of rGM-CSF to ongoing antimicrobial therapy and subsequent neutrophil recovery; however, the role of rGM-CSF in management of established infection in patients with neutropenia remains to be more thoroughly investigated.
The pharmacokinetic properties of rGM-CSF depend on the route of administration. After intravenous administration, serum levels decline rapidly with a half-life of distribution (t1/2α) of 5 to 15 minutes and half-life of elimination (t1/2β) of 1.5 to 2 hours. Maximum serum concentrations are reached 2 hours after subcutaneous injection, then decline with a t1/2β of 3 hours. Serum levels of rGM-CSF increase with dose and a proposed therapeutic target level of 1 µg/L is maintained for 8 to 22 and 16 hours after administration of 15 µg/kg of rGM-CSF by intravenous bolus and subcutaneous injection, respectively.
Therapeutic Use
The correlation between duration and severity of neutropenia and incidence of serious infection is well established. Administration of rGM-CSF to bone marrow transplant recipients is aimed at reducing morbidity in the early post-transplant period by shortening the duration of agranulocytosis. Intravenous administration of rGM-CSF up to 16 µg/kg/day (approximately 640 µg/m2/day) is well tolerated, and when begun within 24 hours of autologous marrow infusion produces the earlier appearance of > 0.5 × 109/L neutrophils in the peripheral circulation as compared with historical controls. Early studies indicate that treated patients have a lower incidence of culture-proven bacteraemia, and recent reports, some preliminary, of placebo-controlled and randomised trials confirm that patients with nonHodgkin’s lymphoma or acute lymphocytic leukaemia who receive rGM-CSF 250 µg/m2 by daily 2-hour infusion for 21 days or more post transplantation, have significant reductions in duration of infectious episodes, antibiotic administration and hospitalisation.
More limited data support a similar acceleration of neutrophil recovery in allogeneic bone marrow transplant recipients treated with rGM-CSF, with no apparent effect on the incidence or severity of graft-versus-host disease.
rGM-CSF is less effective in patients in whom progenitor cell numbers are reduced by chemical purging of the marrow whether administered immediately after marrow infusion or when used as salvage therapy in patients with graft failure. A substantial proportion of patients with failure of autologous or allogeneic bone marrow grafts respond to prompt administration of rGM-CSF after diagnosis of graft failure, with an increase in absolute neutrophil count and bone marrow cellularity. In 1 study of 37 such patients, overall survival was significantly improved compared with historical controls.
rGM-CSF increases the number of progenitor cells in peripheral circulation and, either alone or in combination with cyclophosphamide, facilitates the harvest of stem cells by apheresis for subsequent transplantation.
Similar to the effect seen after myeloablative therapy and marrow transplantation, rGM-CSF accelerates neutrophil recovery following cytoreductive chemotherapy in patients with nonhaematological malignancies. Less frequent and less severe mucositis was also observed in rGM-CSF-treated versus control patients in several studies. Importantly, adjunctive use of rGM-CSF facilitated delivery of planned cycles of high or escalated doses of antineoplastic drugs although the value of such chemotherapy regimens remains to be proven.
There has been no evidence to date that rGM-CSF increases the rate of relapse of patients with haematological malignancies when administered after myeloablative therapy and bone marrow transplantation or, in patients with acute myelogenous leukaemia, after induction therapy. Use of rGM-CSF to recruit quiescent leukaemic blast cells into S phase prior to chemotherapy is under investigation.
rGM-CSF has been investigated in various disorders of haematopoiesis. A substantial number of adults and children with refractory aplastic anaemia respond to treatment with increases in bone marrow cellularity and peripheral blood granulocyte count; however, the response is generally not sustained after withdrawal of rGM-CSF. Elevation of neutrophil counts may not occur in patients with long-standing and severe aplasia; however, beneficial stimulation of macrophage function may still occur. Generally, rGM-CSF induces eosinophilia without correcting the neutropenia in patients with congenital neutropenic conditions.
In myelodysplasia, rGM-CSF is capable of increasing the neutrophil count in a proportion of patients for the duration of administration. Caution is appropriate in administering this drug to patients with high (> 14% blasts) initial leukaemic burdens or with chronic myelomonocytic leukaemia in view of the potential for rGM-CSF to stimulate the leukaemic clone and precipitate acute leukaemia. Despite this concern, encouraging preliminary results from a trial with rGM-CSF (3 µg/kg/day by subcutaneous injection) and observation-only treatment groups suggest that, after > 6 months, the rate of transformation to acute leukaemia is similar in both groups but that rGM-CSF recipients have a sustained increase in neutrophil counts and an associated reduction in infection rate.
rGM-CSF 1 to 5 µg/kg/day by subcutaneous injection ameliorates leucopenia associated with HIV infection and corrects zidovudine (azidothymidine)-induced neutropenia without affecting the disease course as determined by p24 antigen levels, CD4: CD8 ratios and recovery of HIV from mononuclear cells. Similar dosages ameliorate myelosuppression induced by ganciclovir in the treatment of AIDS-associated cytomegalovirus retinitis and by the combination of zidovudine and interferon-α in treating Kaposi’s sarcoma.
A trilineage response to rGM-CSF has been seen occasionally (e.g. some children with aplastic anaemia and some patients with myelodysplasia). Disease-or drug-induced anaemia or thrombocytopenia is generally not improved; however, both significant increases and decreases in platelet count have been reported, and the effect of rGM-CSF on megakaryocytosis and splenic phagocyte function require clarification. The combination of rGM-CSF with other recombinant colony-stimulating factors to expand the lineages stimulated is an exciting future possibility.
Tolerability
At clinically useful dosages rGM-CSF is generally well tolerated. Limited comparison with placebo suggests that the type and incidence of adverse reactions reported are generally similar in both groups with the possible exception of slightly higher incidences of diarrhoea, asthenia, rash and malaise. However, reports from noncomparative and open-label trials indicate that mild to moderate flu-like symptoms (myalgias, bone pain, fatigue and headache) are common with rGM-CSF. Management of patients in whom this agent is indicated may be complicated by rGM-CSF-induced fever and, rarely, by a capillary leak syndrome causing fluid retention and potentially peripheral oedema, pericardial or pleural effusions which necessitate drug withdrawal. Also reported are rash (particularly at sites of subcutaneous injection), and occasional incidents of central venous catheter thrombosis. The occasional report of respiratory distress has led to the recommendation that respiratory symptoms be monitored and caution exercised in patients with preexisting lung disease.
Dosage and Administration
The approved (USA) dosage of yeast-derived rGM-CSF (sargramostim) for myeloid reconstitution after autologous bone marrow transplantation is 250 µg/m2 by daily 2-hour intravenous infusions, beginning 2 to 4 hours after marrow infusion and continued for 21 days. For management of bone marrow transplantation failure or delayed engraftment, the approved (USA) dosage of yeast-derived rGM-CSF is 250 µg/m2/day by 2-hour intravenous infusion. Treatment should be continued for 14 days and, if clinically indicated, may be repeated after 7 days off therapy. A third 14-day course of rGM-CSF at the increased dosage of 500 µg/m2/day by 2-hour infusion may be administered after a further 7 days off therapy. Further dose escalation in non-responding patients is unlikely to be of benefit.
rGM-CSF has also been successfully administered by continuous intravenous infusion and by subcutaneous injection, including self-administration of long term therapy by the subcutaneous route. The optimal route for administration, dose and duration of therapy for indications other than autologous bone marrow transplantation and failure or delay of engraftment have not been established.
Similar content being viewed by others
References
Addison IE, Johnson B, Devereux S, Goldstone AH, Linch DC. Granulocyte-macrophage colony-stimulating factor may inhibit neutrophil migration in vivo. Clinical and Experimental Immunology 76: 149–153, 1989
Aglietta M, Monzeglio C, Piacibello W, Aprá F, Stacchini A, et al. GM-CSF: intravenous versus subcutaneous treatment. Leukemia 4: 523, 1990
Aglietta M, Monzeglio C, Sanavio F, Apra F, Morelli S, et al. In vivo effect of human granulocyte-macrophage colony-stimulating factor on megakaryocytopoiesis. Blood 77: 1191–1194, 1991
Anaisse E, Bodey GP, O’Brien S. GMCSF improves response to amphotericin B in neutropenic patients with systemic mycosis. Abstract 41. Blood 74: 15a, 1989.
Andersson R, Elgefors B, Ridell B, Gisslén M, Kutti J. GM-CSF expands the eosinophilic compartment in chronic idiopathic neutropenia. European Journal of Haematology 44: 315–316, 1990
Antin JH, Smith BR, Holmes W, Rosenthal S. Phase I/II study of recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF) in aplastic anemia and myelodysplastic syndrome. Blood 72: 705–713, 1988
Antman KH. G-CSF and GM-CSF in clinical trials. Yale Journal of Biology and Medicine 63: 387–410, 1990
Antman KH, Griffin J, Elias A, Socinski MA, Ryan L, et al. Effect of recombinant human granulocyte-macrophage colony-stimulating factor on chemotherapy-induced myelosuppression. New England Journal of Medicine 319: 593–598, 1988
Appelbaum FR, Nemunaitis J, Singer JW, Buckner CD, Storb R, et al. Use of recombinant human granulocyte macrophage colony-stimulating factor to speed engraftment and treat graft failure following marrow transplantation in man. Haematology and Blood Transfusion 33: 736–740, 1990
Ardizzoni A, Sertoli MR, Corcione A, Pennucci MC, Baldini E, et al. Accelerated chemotherapy with or without GM-CSF for small cell lung cancer: a non-randomised pilot study. European Journal of Cancer 26: 937–941, 1990
Arnaout MA, Wang EA, Clark SC, Sieff CA. Human recombinant granulocyte-macrophage colony-stimulating factor increases cell-to-cell adhesion and surface expression of adhesion-promoting surface glycoproteins on mature granulocytes. Journal of Clinical Investigation 78: 597–601, 1986
Arning M, Kliche KO, Schneider W. GM-CSF therapy and capillary-leak syndrome. Annals of Hematology 62: 83, 1991
Atkinson YH, Lopez AF, Marasco WA, Lucas CM, Wong GG. Recombinant human granulocyte-macrophage colony-stimulating factor (rH GM-CSF) regulates f Met-Leu-Phe receptors on human neutrophils. Immunology 64: 519–525, 1988
Baldwin GC, Gasson JC, Quan SG, Fleischmann J, Weisbart R, et al. Granulocyte-macrophage colony-stimulating factor enhances neutrophil function in acquired immunodeficiency syndrome patients. Proceedings of the National Academy of Sciences of the United States of America 85: 2763–2766, 1988
Baldwin GC, Gasson JC, Kaufman SE, Quan SG, Williams RE, et al. Non-hematopoietic tumor cells express functional GMCSF receptors. Blood 73: 1033–1037, 1989a
Baldwin GC, Fuller ND, Roberts RL, Ho DD, Golde DW. Granulocyte- and granulocyte-macrophage colony-stimulating factors enhance neutrophil cytotoxicity toward HIV-infected cells. Blood 74: 1673–1677, 1989b
Bandini G, Rosti G, Cavo M, Albertazzi L, Tura S. Acceleration of autologous hematopoietic recovery by GM-CSF after allograft rejection. Haematologia 74: 627–628, 1989
Barlogie B, Jagannath S, Dixon DO, Cheson B, Smallwood L, et al. High-dose melphalan and granulocyte-macrophage colony-stimulating factor for refractory multiple myeloma. Blood 76: 677–680, 1990
Begley CG, Metcalf D, Nicola NA. Purified colony stimulating factors (G-CSF and GM-CSF) induce differentiation in human HL60 leukemic cells with suppression of clonogenicity. International Journal of Cancer 39: 99–105, 1987a
Begley CG, Metcalf D, Nicola NA. Primary human myeloid leukemia cells: comparative responsiveness to proliferative stimulation by GM-CSF or G-CSF and membrane expression of CSF receptors. Leukemia 1: 1–8, 1987b
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DAG, et al. Proposal for classification of the myelodysplastic syndromes. British Journal of Haematology 51: 189–199, 1982
Berdel WE, Danhauser-Riedl S, Steinhauser G, Rastetter J. Stimulation of clonal growth of human colorectal tumor cells by IL-3 and GM-CSF. Modulation of 5-FU cytotoxicity by GM-CSF. Onkologie 13: 437–443, 1990
Bermudez LEM, Young LS. Recombinant granulocyte-macrophage colony-stimulating factor activates human macrophages to inhibit growth or kill Mycobacterium avium complex. Journal of Leukocyte Biology 48: 67–73, 1990
Bettelheim P, Muhm M, Valent P, Geissler K, Andreeff M, et al. GM-CSF in combination with cytotoxic chemotherapy in AML patients. Bone Marrow Transplantation 6 (Suppl. 1): 127–130, 1990
Bhalla K, Birkhofer M, Arlin Z, Grant S, Lutzky J, et al. Effect of recombinant GM-CSF on the metabolism of cytosine arabinoside in normal and leukemic human bone marrow cells. Leukemia 2: 810–813, 1988
Bhalla K, Birkhofer M, Grant S, Graham G. The effect of recombinant human granulocyte-macrophage colony-stimulating factor (rGM-CSF) on 3′-Azido-3′-deoxythymidine (AZT)-mediated biochemical and cytotoxic effects on normal human myeloid progenitor cells. Experimental Hematology 17: 17–20, 1989
Biesma B, de Vries EGE, Willemse PHB, Sluiter WJ, Postmus PE, et al. Efficacy and tolerability of recombinant human granulocyte-macrophage colony-stimulating factor in patients with chemotherapy-related leukopenia and fever. European Journal of Cancer 26: 932–936, 1990
Blazar BR, Kersey JH, McGlave PB, Vallera DA, Lasky LC, et al. In vivo administration of recombinant human granulocyte/macrophage colony-stimulating factor in acute lymphoblastic leukemia patients receiving purged autografts. Blood 73: 849–857, 1989
Bleiberg I, Riklis I, Fabian I. Enhanced resistance of bone marrow transplanted mice to bacterial infection induced by recombinant granulocyte-macrophage colony-stimulating factor. Blood 75: 1262–1266, 1990
Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Annals of Internal Medicine 64: 328–340, 1966
Bot FJ, van Eijk L, Schipper P, Löwenberg B. Human granulocyte-macrophage colony-stimulating factor (GM-CSF) stimulates immature marrow precursors but no CFU-GM, CFU-G, or CFU-M. Experimental Hematology 17: 292–295, 1989
Bot FJ, van Ejik L, Schipper P, Backx B, Löwenberg B. Synergistic effects between GM-CSF and G-CSF or M-CSF on highly enriched human marrow progenitor cells. Leukemia 4: 325–328, 1990
Brandwein JM, Nayar R, Baker MA, Sutton DMC, Scott JG, et al. GM-CSF therapy for delayed engraftment after autologous bone marrow transplantation. Experimental Hematology 19: 191–195, 1991
Brandt SJ, Peters WP, Atwater SK, Kurtzberg J, Borowitz MJ, et al. Effect of recombinant human granulocyte-macrophage colony-stimulating factor on hematopoietic reconstitution after high-dose chemotherapy and autologous bone marrow transplantation. New England Journal of Medicine 318: 869–876, 1988
Braun DP, Siziopikou KP, Casey LC, Harris JE. The in vitro development of cytotoxicity in response to granulocyte/macrophage-colony-stimulating factor or interferon γ in the peripheral blood monocytes of patients with solid tumors: modulation by arachidonic acid metabolic inhibitors. Cancer Immunology Immunotherapy 32: 55–61, 1990
Briddell RA, Hoffman R. Cytokine regulation of the human burst-forming unit-megakaryocyte. Blood 76: 516–522, 1990
Brito-Babapulle F, Lord JAD, Whitmore DN. Treatment of RAEB-t with intensive chemotherapy and GM-CSF. Leukemia Research 13: 605–607, 1989
Broxmeyer HE, Cooper S, Williams DE, Hangoc G, Gutterman JU, et al. Growth characteristics of marrow hematopoietic progenitor/precursor cells from patients on a phase I clinical trial with purified recombinant human granulocyte-macrophage colony-stimulating factor. Experimental Hematology 16: 594–602, 1988
Broxmeyer HE, Cooper S, Vadhan-Raj S. Cell cycle status of erythroid (BFU-E) progenitor cells from the bone marrows of patients on a clinical trial with purified recombinant human granulocyte-macrophage colony-stimulating factor. Experimental Hematology 17: 455–459, 1989
Broxmeyer HE, Cooper S, Lu L, Hangoc G, Anderson D, et al. Effect of murine mast cell growth factor (c-kit) proto-oncogene ligand) on colony formation by human marrow hematopoietic progenitor cells. Blood 77: 2142–2149, 1991
Budel LM, Elbaz O, Hoogerbrugge H, Delwel R, Mahmoud LA, et al. Common binding structure for granulocyte macrophage colony-stimulating factor and interleukin-3 on human acute myeloid leukemia cells and monocytes. Blood 75: 1439–1445, 1990
Buechner T, Hiddemann W, Koenigsmann M, Zuehlsdorf M, Woermann B, et al. Recombinant human GM-CSF following chemotherapy in high-risk AML. Bone Marrow Transplant 6: 131–134, 1990
Butturini A, Gale RP, Lopes DM, Cunha CB, Ho WG. Use of recombinant granulocyte-macrophage colony stimulating factor in the Brazil radiation accident. Lancet 8609: 471–474, 1988
Cairo MS, van de Ven C, Toy C, Mauss D, Sender L. Recombinant human granulocyte-macrophage colony-stimulating factor primes neonatal granulocytes for enhanced oxidative metabolism and chemotaxis. Pediatric Research 26: 395–399, 1989
Cannistra SA, Rambaldi A, Spriggs DR, Herrmann F, Kufe D, et al. Human granulocyte-macrophage colony-stimulating factor induces expression of the tumour necrosis factor gene by the U937 cell line and by normal human monocytes. Journal of Clinical Investigation 79: 1720–1728, 1987
Cannistra SA, Groshek P, Griffin J. Granulocyte-macrophage colony-stimulating factor enhances the cytotoxic effects of cytosine arabinoside in acute myeloblastic leukemia and in the myeloid blast crisis phase of chronic myeloid leukemia. Leukemia 3: 328–334, 1989
Cannistra SA, Groshek P, Garlick R, Miller J, Griffin J. Regulation of surface expression of the granulocyte/macrophage colony-stimulating factor receptor in normal human myeloid cells. Proceedings of the National Academy of Science of the United Sates of America 87: 93–97, 1990a
Cannistra SA, Koenigsmann M, DiCarlo J, Groshek P, Griffin J. Differentiation-associated expression of two functionally distinct classes of granulocyte-macrophage colony-stimulating factor receptors by human myeloid cells. Journal of Biological Chemistry 265: 12656–12663, 1990b
Cannistra SA, DiCarlo J, Groshek P, Kanakura Y, Berg D, et al. Simultaneous administration of granulocyte-macrophage colony-stimulating factor and cytosine arabinoside for the treatment of relapsed acute myeloid leukemia. Leukemia 5: 230–238, 1991
Cantrell MA, Anderson D, Cerretti DP, Price V, McKereghan K, et al. Cloning, sequence, and expression of a human granulocyte/macrophage colony-stimulating factor. Proceedings of the National Academy of Sciences of the United States of America 82: 6250–6254, 1985
Caracciolo D, Shirsat N, Wong GG, Lange B, Clark S, et al. Recombinant human macrophage colony-stimulating factor (M-CSF) requires subliminal concentrations of granulocyte/macrophage (GM)-CSF for optimal stimulation of human macrophage colony formation in vitro. Journal of Experimental Medicine 166: 1851–1860, 1987
Caracciolo D, Clark S, Rovera G. Differential activity of recombinant colony-stimulating factors in supporting proliferation of human peripheral blood and bone marrow myeloid progenitors in culture. British Journal of Haematology 72: 306–311, 1989
Caracciolo D, Pannocchia A, Treves S, Ghigo D, Gallo E, et al. Role of Na+/H+ exchange in the granulocyte-macrophage colony-stimulating factor-dependent growth of a leukemic cell line. Journal of Cellular Physiology 143: 133–139, 1990
Carlo-Stella C, Mangoni L, Almici C, Frassoni F, Fiers W, et al. Growth of CD34+ acute myeloblastic leukemia colony-forming cells in response to recombinant hematopoietic growth factors. Leukemia 4: 561–566, 1990
Caulfield JP, Hein A, Rothenburg ME, Owen WF, Soberman RJ, et al. A morphometric study of normodense and hypodense human eosinophils that are derived in vivo and in vitro. American Journal of Pathology 137: 27–41, 1990
Cebon J, Dempsey P, Fox R, Kannourakis G, Bonnern E, et al. Pharmacokinetics of human granulocyte-macrophage colony-stimulating factor using a sensitive immunoassay. Blood 72: 1340–1347, 1988
Cebon JS, Bury RW, Lieschke GJ, Morstyn G. The effects of dose and route of administration on the pharmacokinetics of granulocyte-macrophage colony-stimulating factor. European Journal of Cancer 26: 1064–1069, 1990a
Cebon J, Nicola N, Ward M, Gardner I, Dempsey P, et al. Granulocyte-macrophage colony-stimulating factor from human lymphocytes. Journal of Biological Chemistry 265: 4483–4491, 1990b
Champlin RE, Nimer SD, Ireland P, Oette DH, Golde DW. Treatment of refractory aplastic anemia with recombinant human granulocyte-macrophage-colony-stimulating factor. Blood 73: 694–699, 1989
Chiba S, Tojo A, Kitamura T, Urabe A, Miyazono K, et al. Characterization and molecular features of the cell surface receptor for human granulocyte-macrophage colony-stimulating factor. Leukemia 4: 29–36, 1990
Clark SC, Kamen R. The human hematopoietic colony-stimulating factors. Science 236: 1229–1237, 1987
Coleman DL, Chodakewitz JA, Bartiss AH, Mellors JW. Granulocyte-macrophage colony-stimulating factor enhances selective effector functions of tissue-derived macrophages. Blood 72: 573–578, 1988
Corey SJ, Rosoff PM. Granulocyte-macrophage colony-stimulating factor primes neutrophils by activating a pertussis toxin-sensitive G protein not associated with phosphatidylinositol turnover. Journal of Biological Chemistry 264: 14165–14171, 1989
Daghistani D, Jimenez JJ, Toledano SR, Cirocco RE, Yunis AA. Congenital neutropenia: a case study. American Journal of Pediatric Hematology/Oncology 12: 210–214, 1990
Dahinden CA, Kurimoto Y, Wirthmüller U. Growth factors, lipid mediators and effector cells. Journal of Lipid Mediators 2: S129–S136, 1990
Davey Jr RT, Davey VJ, Metcalf JA, Kovacs JA et al. A phase I/II trial of zidovudine, interferon-alpha, and granulocyte-macrophage colony-stimulating factor in the treatment of human immunodeficiency virus type I infection. Journal of Infectious Diseases 164: 43–52, 1991
Dedhar S, Gaboury L, Galloway P, Eaves C. Human granulocyte-macrophage colony-stimulating factor is a growth factor active on a variety of cell types of nonhemopoietic origin. Proceedings of the National Academy of Sciences of the United States of America 85: 9253–9257, 1988
Delmer A, Karmochkine M, Cadiou M, Gerhartz H, Zittoun R. Recurrent spleen enlargement during cyclic granulocyte-macrophage colony-stimulating factor therapy for myelodysplastic syndrome. American Journal of Hematology 34: 73–74, 1990
Delwel R, Salem M, Pellens C, Dorssers L, Wagemaker G, et al. Growth regulation of human acute myeloid leukemia: effects of five recombinant hematopoietic factors in a serum-free culture system. Blood 72: 1944–1949, 1988
Denis M, Ghadirian E. Granulocyte-macrophage colony-stimulating factor restricts growth of tubercle bacilli in human macrophages. Immunology Letters 24: 203–206, 1990
Devereaux S, Linch DC, Gribben JG, McMillan A, Patterson K, et al. GM-CSF accelerates neutrophil recovery after autologous bone marrow transplantation for Hodgkin’s disease. Bone Marrow Transplantation 4: 49–54, 1989
DeVries EGE, Biesma B, Willemse PHB, Mulder NH, Stern AC, et al. A double-blind placebo-controlled study with granulocyte-macrophage colony-stimulating factor during chemotherapy for ovarian carcinoma. Cancer Research 51: 116–122, 1991a
DeVries EGE, Willemse PHB, Biesma B, Stern AC, Limburg PC, et al. Flare-up of rheumatoid arthritis during GM-CSF treatment after chemotherapy. Correspondence. Lancet 338: 517–518, 1991b
Dippold WG, Klingel R, Kerlin M, Schwaeble W, Büschenfelde K-HMZ. Stimulation of pancreas and gastric carcinoma cell growth by interleukin 3 and granulocyte-macrophage colony-stimulating factor. Gastroenterology 100: 1338–1344, 1991
Djeu JY, Widen R, Blanchard DK. Susceptibility of monocytes to lymphokine-activated killer cell lysis: effect of granulocyte-macrophage colony-stimulating factor and interleukin-3. Blood 73: 1264–1271, 1989
Donahue RE, Wang EA, Stone DK, Kamen R, Wong GG, et al. Stimulation of haematopoiesis in primates by continuous infusion of recombinant human GM-CSF. Nature 321: 872–875, 1986
Drings P, Fischer JR. Biology and clinical use of GM-CSF in lung cancer. Lung 168 (Suppl.): 1059–1068, 1990
Edmonson JH, Long HJ, Jeffries JA, Buckner JC, Colonotero G, et al. Amelioration of chemotherapy-induced thrombocytopenia by GM-CSF: apparent dose and schedule dependency. Journal of the National Cancer Institute 81: 510–512, 1989
Edwards SW, Holden CS, Humphreys JM, Hart CA. Granulocyte-macrophage colony-stimulating factor (GM-CSF) primes the respiratory burst and stimulates protein biosynthesis in human neutrophils. FEBS Letters 256: 62–66, 1989
Egli E, Hofer S, Greminger P, Rhyner K. Kombinierte GM-CSF- und Erythropoietintherapie bei Myelodysplastischem Syndrom. Schweizerische Medizinische Wochenschrift 119: 1777–1780, 1989
Elliott MJ, Vadas MA, Cleland LG, Gamble JR, Lopez AF. IL-3 and granulocyte-macrophage colony-stimulating factor stimulate two distinct phases of adhesion in human monocytes. Journal of Immunology 145: 167–176, 1990
Emminger W, Emminger-Schmidmeier W, Peters C, Susani M, Hawliczek R, et al. Capillary leak syndrome during low dose granulocyte-macrophage colony-stimulating factor (rhGM-CSF) treatment of a patient in a continuous febrile state. Blut 61: 219–221, 1990
Estey EH, Kurzrock R, Talpaz M, McCredie KB, O’Brien S, et al. Effects of low doses of recombinant human granulocyte-macrophage colony stimulating factor (GM-CSF) in patients with myelodysplastic syndromes. British Journal of Haematology 77: 291–295, 1991
Estey EH, Kantarjian HM, Beran M, McCredie KB, Keating MJ, et al. Treatment of poor-prognosis, newly diagnosed acute myelogenous leukemia with high-dose cytosine arabinoside (Ara-C) and rHUGM-CSF. Haematology and Blood Transfusion 33: 732–735, 1990
Evans JPM, Mire-Sluis AR, Hoffbrand AV, Wickremasinghe RG. Binding of G-CSF, GM-CSF, tumor necrosis factor-α, and γ-interferon to cell surface receptors on human myeloid leukemia cells triggers rapid tyrosine and serine phosphorylation of a 75-Kd protein. Blood 75: 88–95, 1990
Fabian I, Baldwin GC, Golde DW. Biosynthetic granulocyte-macrophage colony-stimulating factor enhances neutrophil cytotoxicity toward human leukemia cells. Leukemia 1: 613–617, 1987
Fabian I, Kletter Y, Bleiberg I, Gadish M, Naparsteck E, et al. Effect of exogenous recombinant granulocyte-macrophage colony-stimulating factor on neutrophil function following allogenic bone marrow transplantation. Experimental Hematology 19: 868–873, 1991
Faisal M, Cumberland W, Champlin R, Fahey JL. Effect of recombinant human granulocyte-macrophage colony-stimulating factor administration on the lymphocyte subsets of patients with refractory aplastic anemia. Blood 76: 1580–1585, 1990
Farmer KL, Kurzrock R, Gutterman JV, Duvic M. Necrotizing vasculitis at granulocyte-macrophage-colony-stimulating factor injection sites. Archives of Dermatology 126: 1243–1244, 1990
Ferrero D, Tarella C, Badoni R, Caracciolo D, Bellone G, et al. Granulocyte-macrophage colony-stimulating factor requires interaction with accessory cells or granulocyte-colony stimulating factor for full stimulation of human myeloid progenitors. Blood 73: 402–405, 1989
Fischer H, Frosch S, Reske K, Reske-Kunz AB. Granulocyte-macrophage colony-stimulating factor activates macrophages derived from bone marrow cultures to synthesis of MHC class II molecules and to augment antigen presentation function. Journal of Immunology 141: 3882–3888, 1988
Fischer T, Wiegmann K, Böttinger H, Morens K, Burmester, et al. Regulation of IFN-γ-receptor expression in human monocytes by granulocyte-macrophage colony-stimulating factor. Journal of Immunology 145: 2914–2919, 1990
Fischl M, Uttamchandani R, Gagnon S, Thompson L, Santiago S. Phase I study of interferon alpha-2b intron zidovudine and rGM-CSF in patients with AIDS-associated Kaposi’s sarcoma. Presented at the fifth International Conference on AIDS: Montreal, June 4–9, 1989
Fleischmann J, Golde DW, Weisbart RH, Gasson JC. Granulocyte-macrophage colony-stimulating factor enhances phagocytosis of bacteria by human neutrophils. Blood 68: 708–711, 1986
Fouillard L, Gorin NC, Laporte JPh, Douay L, Isnard F, et al. Recombinant human granulocyte-macrophage colony-stimulating factor plus the BEAM regimen instead of autologous bone marrow transplantation. Correspondence. Lancet 8650: 1460, 1989a
Fouillard L, Gorin NC, Laporte JPh, Eugene-Jolchine I, Isnard F, et al. GM-CSF and ganciclovir for cytomegalovirus infection after autologous bone-marrow transplantation. Correspondence. Lancet 2: 1273, 1989b
Foulke RS, Marshall MH, Trotta PP, Von Hoff DD. In vitro assessment of the effects of granulocyte-macrophage colony-stimulating factor on primary human tumors and derived lines. Cancer Research 50: 6264–6267, 1990
Frenck Jr RW, Buescher ES, Vadhan-Raj S. The effects of recombinant human granulocyte-macrophage colony stimulating factor on in vitro cord blood granulocyte function. Pediatric Research 26: 43–48, 1989
Freund MRF, Luft S, Schober C, Heussner P, Schrezenmaier H, et al. Differential effect of GM-CSF and G-CSF in cyclic neutropenia. Lancet 336: 313, 1990
Fujisawa T, Abu-Ghazaleh R, Kita H, Sanderson CJ, Gleich GJ. Regulatory effect of cytokines on eosinophil degranulation. Journal of Immunology 144: 642–646, 1990
Furman WL, Fairclough DL, Huhn RD, Pratt CB, Stute N, et al. Therapeutic effects and pharmacokinetics of recombinant human granulocyte-macrophage colony-stimulating factor in childhood cancer patients receiving myelosuppressive chemotherapy. Journal of Clinical Oncology 9: 1022–1028, 1991
Gamble JR, Rand TH, Lopez AF, Clark-Lewis I, Vadas MA. Heterogeneity of recombinant granulocyte-macrophage colony-stimulating factor-mediated enhancement of neutrophil adherence to endothelium. Experimental Hematology 18: 897–902, 1990
Ganser A, Ottmann OG, Erdmann H, Schulz G, Hoelzer D. The effect of recombinant human granulocyte-macrophage colony-stimulating factor on neutropenia and related morbidity in chronic severe neutropenia. Annals of Internal Medicine 111: 887–892, 1989a
Ganser A, Völkers B, Greher J, Ottmann OG, Walther F, et al. Recombinant human granulocyte-macrophage colony-stimulating factor in patients with myelodysplastic syndromes — a phase I/II trial. Blood 73: 31–37, 1989b
Gasson JC, Fraser JK, Nimer SD. Human granulocyte-macrophage colony-stimulating factor (GM-CSF): regulation of expression. Hematopoietic Growth Factors in Transfusion Medicine: 27–41, 1990
Gattringer C, Thaler J, Drach J, Micksche M, Huber H. GM-CSF treatment in aplasia after cytotoxic therapy. Onkologie 12: 16–18, 1989
Geissler D, Niederwieser D, Aulitzky WE, Tilg H, Grünewald K, et al. Serum colony stimulating factors in patients undergoing bone marrow transplantation: enhancing effect of recombinant human GM-CSF. Behring Institute Mitteilungen 83: 289–300, 1988
Geissler K, Harrington M, Srivastava C, Leemhuis T, Tricot G, et al. Effects of recombinant human colony stimulating factors (CSF) (granulocyte-macrophage CSF, granulocyte CSF, and CSF-1) on human monocyte/macrophage differentiation. Journal of Immunology 143: 140–146, 1989
Geissler K, Tricot G, Grimm G, Siostrzonek P, Broxmeyer H. Recombinant human colony stimulating factor-granulocyte/macrophage and granulocyte, but not macrophage induce the development of a respiratory burst in primary human myeloid leukemic cells in vitro. Blut 59: 226–230, 1989b
Gerhartz HH, Visani G, Delmer A, Zwierzina H, Ribeiro M, et al. Low-dose Ara-C plus granulocyte-macrophage colony-stimulating factor for the treatment of myelodysplastic syndromes. Bone Marrow Transplantation 5: 36–37, 1989
Gianni AM, Bregni M, Siena S, Orazi A, Stern AC, et al. Recombinant human granulocyte-macrophage colony-stimulating factor reduces hematologic toxicity and widens clinical applicability of high-dose cyclophosphamide treatment in breast cancer and non-Hodgkin’s lymphoma. Journal of Clinical Oncology 8: 768–778, 1990a
Gianni AM, Bregni M, Stern AC, Bonadonna G, Siena S, et al. Granulocyte-macrophage colony-stimulating factor to harvest circulating haemopoietic stem cells for autotransplantation. Lancet 8663: 580–584, 1989
Gianni AM, Tarella C, Siena S, Bregni M, Boccadoro M, et al. Durable and complete hematopoietic reconstitution after autografting of rhGM-CSF exposed peripheral blood progenitor cells. Bone Marrow Transplantation 6: 143–145, 1990b
Gillis S, Urdal DL, Clevenger W, Klinke R, Sassenfeld H, et al. Production of recombinant human colony stimulating factors in yeast. Behring Institute Mitteilungen 83: 1–7, 1988
Gomez-Cambronero J, Yamazaki M, Metwally F, Melski TFP, Bonak VA, et al. Granulocyte-macrophage colony-stimulating factor and human neutrophils: Role of guanine nucleotide regulatory proteins. Proceedings of the National Academy of Science of the United States of America 86: 3569–3573, 1989
Gonzales-Chambers R, Rosenfeld C, Winkelstein A, Dameshek L. Eosinophilia resulting from administration of recombinant granulocyte-macrophage colony-stimulating factor (rhGM-CSF) in a patient with T-lymphoproliferative disease. American Journal of Hematology 36: 157–159, 1991
Gordon-Smith EC, Yandle A, Milne A, Speck B, Marmont A, et al. Randomized placebo controlled study of RH-GM-CSF following ALG in the treatment of aplastic anaemia. Bone Marrow Transplantation 7 (Suppl. 2): 78–80, 1991
Gorin NC, Coiffier B, Hayat M, Philip T, Vernant JP, et al. RHU GM-CSF shortens aplasia duration after ABMT in non-Hodgkin’s lymphoma: a randomized placebo-controlled double-blind study. Bone Marrow Transplantation 7 (Suppl. 2): 82, 1991
Grabstein KH, Urdal DL, Tushinski RJ, Mochizuki DY, Price VL, et al. Induction of macrophage tumoricidal activity by granulocyte-macrophage colony-stimulating factor. Science 232: 506–508, 1986
Gribben JG, Devereux S, Thomas NS, Keim M, Jones HM, et al. Development of antibodies to unprotected glycosylation sites on recombinant human GM-CSF. Lancet 1: 434–437, 1990
Griffin J, Spertini O, Ernst TJ, Belvin MP, Levine HB, et al. Granulocyte-macrophage colony-stimulating factor and other cytokines regulate surface expression of the leukocyte adhesion molecule-1 on human neutrophils, monocytes, and their precursors. Journal of Immunology 145: 576–584, 1990
Gronski P, Badziong W, Habermann P, List W, Müllner H, et al. E. coli derived human granulocyte-macrophage colony-stimulating factor (rh GM-CSF) available for clinical trials. Behring Institute Mitteilungen 83: 246–249, 1989
Groopman JE, Mitsuyasu RT, DeLeo MJ, Oette DH, Golde DW. Effect of recombinant human granulocyte-macrophage colony-stimulating factor on myelopoiesis in the acquired immunodeficiency syndrome. New England Journal of Medicine 317: 593–598, 1987
Grossberg HS, Bonnern EM, Buhles Jr WC. GM-CSF with ganciclovir for the treatment of CMV retinitis in aids. New England Journal of Medicine 320(23): 1560, 1989
Guinan EC, Sieff CA, Oette DH, Nathan D. A phase I/II trial of recombinant granulocyte-macrophage colony-stimulating factor for children with aplastic anemia. Blood 76: 1077–1082, 1990
Gurney H, Crowther D. Hemopoietic growth factors: their clinical role. Drugs of the Future 15: 582–596, 1990
Haak-Frendscho M, Arai N, Arai K-i, Baeza ML, Finn A, et al. Human recombinant granulocyte-macrophage colony-stimulating factor and interleukin 3 cause basophil histamine release. Journal of Clinical Investigation 82: 17–20, 1988
Haas R, Ho AD, Bredthauer U, Cayeux S, Egerer G, et al. Successful autologous transplantation of blood stem cells mobilized with recombinant human granulocyte-macrophage colony-stimulating factor. Experimental Hematology 18: 94–98, 1990
Haase D, Fonatsch C. Karyotype and in vitro-response to GM-CSF. Analysis of bone marrow cultures in leukemia, myelodysplasia and aplastic anemia. Blut 60: 192–197, 1990a
Haase D, Fonatsch C. Monosomy 7 provides a proliferative advantage for leukemic cells under incubation with GM-CSF in vitro. Correspondence. Blut 61: 322–323, 1990b
Hammer SM, Gillis JM. Synergistic activity of granulocyte-macrophage colony-stimulating factor and 3′-azido-3′deoxythymidine against human immunodeficiency virus in vitro. Antimicrobial Agents and Chemotherapy 31: 1046–1050, 1987
Hammer SM, Gillis JM, Groopman JE, Rose RM. In vitro modification of human immunodeficiency virus infection by granulocyte-macrophage colony-stimulating factor and interferon. Proceedings of the National Academy of Sciences of the United States of America 83: 8734–8738, 1986
Hammer SM, Gillis JM, Pinkston P, Rose RM. Effect of zidovudine and granulocyte-macrophage colony-stimulating factor on human immunodeficiency virus replication in alveolar macrophages. Blood 75: 1215–1219, 1990
Hancock WW, Pleau ME, Kobzik L. Recombinant granulocyte-macrophage colony-stimulating factor down-regulates expression of IL-2 receptor on human mononuclear phagocytes by induction of prostaglandin. Journal of Immunology 140: 3021–3025, 1988
Handman E, Burgess AW. Stimulation by granulocyte-macrophage colony-stimulating factor of Leishmania tropica killing by macrophages. Journal of Immunology 122: 1134–1137, 1979
Hardy WD. Combined ganciclovir and recombinant human granulocyte-macrophage colony-stimulating factor in the treatment of cytomegalovirus retinitis in AIDS patients. Journal of Acquired Immune Deficiency Syndromes 4 (Suppl. 1): 22–28, 1991
Hart PH, Whitty GA, Piccoli DS, Hamilton JA. Synergistic activation of human monocytes by granulocyte-macrophage colony-stimulating factor and IFN-γ. Increased TNF-α but not IL-1 activity. Journal of Immunology 141: 1516–1521, 1988
Hassan HT, Zyada LE, Ragab MH, Rees JKH. Synergistic interactions between recombinant human interleukin-3, GM-CSF and G-CSF in normal human marrow granulocyte-macrophage colony formation. Cell Biology International Reports 15: 211, 1991
Hazenberg BPC, Van Leeuwen MA, Van Rijswijk, Stern AC, Vellenga E. Correction of granulocytopenia in Felty’s syndrome by granulocyte-macrophage colony-stimulating factor. Simultaneous induction of interleukin-6 release and flare-up of the arthritis. Blood 74: 2769–2773, 1989
Henderson GS. Hormones of the 1990s? The colony-stimulating factors — part 1: haematology and development. Australian Journal of Hospital Pharmacy 20: 368–370, 1990
Herrmann F, Lindemann A, Klein H, Lübbert M, Schulz G, et al. Effect of recombinant human granulocyte-macrophage colony-stimulating factor in patients with myelodysplastic syndrome with excess blasts. Leukemia 3: 335–338, 1989a
Herrmann F, Schulz G, Lindemann A, Meyenburg W, Oster W, et al. Hematopoietic responses in patients with advanced malignancy treated with recombinant human granulocyte-macrophage colony-stimulating factor. Journal of Clinical Oncology 7: 159–167, 1989b
Herrmann F, Ganser A, Lindemann A, Schulz G, Lübbert M, et al. Stimulation of granulopoiesis in patients with malignancy by recombinant human granulocyte-macrophage colony-stimulating factor: assessment of two routes of administration. Journal of Biological Response Modifiers 9: 475–479, 1990a
Herrmann F, Schulz G, Wieser M, Kolbe K, Nicolay U, et al. Effect of granulocyte-macrophage colony-stimulating factor on neutropenia and related morbidity induced by myelotoxic chemotherapy. American Journal of Medicine 88: 619–624, 1990b
Heufler C, Koch F, Schuler G. Granulocyte-macrophage colony-stimulating factor and interleukin 1 mediate the maturation of murine epidermal Langerhans cells into potent immunostimulatory dendritic cells. Journal of Experimental Medicine 167: 700–705, 1988
Ho AD, Del Valle F, Haas R, Engelhard M, Hiddemann W, et al. Sequential studies on the role of mitoxantrone, high-dose cytarabine, and recombinant human granulocyte-macrophage colony-stimulating factor in the treatment of refractory non-hodgkin’s lymphoma. Seminars in Oncology 17: 14–19, 1990a
Ho AD, Haas R, Wulf G, Knauf W, Ehrhardt R, et al. Activation of lymphocytes induced by recombinant human granulocyte-macrophage colony-stimulating factor in patients with malignant lymphoma. Blood 75: 203–212, 1990b
Ho JL, Reed SG, Wick EA, Giordano M. Granulocyte-macrophage and macrophage colony-stimulating factors activate intramacrophage killing of Leishmania mexicana amazonensis. Journal of Infectious Diseases 162: 224–230, 1990c
Ho AD, Haas R, Korbling M, Dietz M, Hunstein W. Utilization of recombinant human GM-CSF to enhance peripheral progenitor cell yield for autologous transplantation. Bone Marrow Transplantation 7: 13–17, 1991
Hoekman K, Wagstaff J, Boven E, van Groeningen CJ, Vermorken JB, et al. A study to determine the maximum tolerable doses (MTD) of adriamycin (ADR) plus cyclophosphamide (CY) in combination with granulocyte-macrophage colony stimulating factor (GMCSF) in patients with breast cancer. Abstract. British Journal of Cancer 62: 8, 1990
Hoelzer D, Ganser A, Ottmann OG, Höffken K, Becher R, et al. Effect of treatment with rhGM-CSF and low-dose cytosine arabinoside on leukemic blast cells in patients with myelodysplastic syndromes. Haematology and Blood Transfusion 33: 763–769, 1990
Hollander AAMJ, Kluin-Nelemans HC, Haak HR, Stern AC, Willemze R, et al. Correction of neutropenia associated with chronic lymphocytic leukaemia following treatment with granulocyte-macrophage colony-stimulating factor. Annals of Hematology 62: 32–34, 1991
Hollingshead LM, Goa KL. Recombinant granulocyte colony-stimulating factor (rG-CSF). A review of its pharmacological properties and prospective role in neutropenic conditions. Drugs 42: 300–330, 1991
Horiguchi J, Warren MK, Kufe D. Expression of the macrophage specific colony-stimulating factor in human monocytes treated with granulocyte-macrophage colony-stimulating factor. Blood 69: 1259–1261, 1987
Horn TD, Burke PJ, Karp JE, Hood AF. Intravenous administration of recombinant human granulocyte-macrophage colony-stimulating factor causes a cutaneous eruption. Archives of Dermatology 127: 49–52, 1991
Howell AL, Stukel TA, Bloomfield CD, Davey FR, Ball ED. Induction of differentiation in blast cells and leukemia colony-forming cells from patients with acute myeloid leukemia. Blood 75: 721–729, 1990
Itoh K, Bessho M, Hirashima K. Effects of recombinant human G-CSF on primary human leukemic cells. Acta Haematologica Japonica 51: 988–995, 1989
Jaswon MS, Khwaja A, Roberts PJ, Jones HM, Linch DC. The effects of rhGM-CSF on the neutrophil respiratory burst when studied in whole blood. British Journal of Haematology 75: 181–187, 1990
Jost LM, Pichert G, Stahel RA. Placebo controlled phase I/II study of subcutaneous GM-CSF in patients with germ cell tumours undergoing chemotherapy. Annals of Oncology 1: 439–442, 1990
Kanakura Y, Druker B, Cannistra SA, Furukawa Y, Torimoto Y, et al. Signal transduction of the human granulocyte-macrophage colony-stimulating factor and interleukin-3 receptors involves tyrosine phosphorylation of a common set of cytoplasmic proteins. Blood 76: 706–715, 1990
Kaplan LD, Kahn JO, Grossberg H, Volberding PA. Chemotherapy with or without granulocyte-macrophage colony stimulating factor (rGM-CSF) in patients with AIDS-associated non-Hodgkin’s lymphoma (NHL). Fifth International Conference on AIDS: Montreal, 334, 1989b
Kaplan SS, Basford RE, Wing EJ, Shadduck RK. The effect of recombinant human granulocyte macrophage colony-stimulating factor on neutrophil activation in patients with refractory carcinoma. Blood 73: 636–638, 1989a
Kapp A, Zeck-Kapp G, Danner M, Luger TA. Human granulocyte-macrophage colony stimulating factor: an effective direct activator of human polymorphonuclear neutrophilic granulocytes. Journal of Investigative Dermatology 91: 49–55, 1988
Karp JE, Burke PJ, Donehower RC. Effects of rhGM-CSF on intracellular ara-C pharmacology in vitro in acute myelocytic leukemia: comparability with drug-induced humoral stimulatory activity. Leukemia 4: 553–556, 1990
Kaufman SE, DiPersio JF, Gasson JC. Effects of human GM-CSF on neutrophil degranulation in vitro. Experimental Hematology 17: 800–804, 1989
Kaushansky K, Shoemaker SG, Alfaro S, Brown C. Hematopoietic activity of granulocyte/macrophage colony-stimulating factor is dependent upon two distinct regions of the molecule: functional analysis based upon the activities of interspecies hybrid growth factors. Proceedings of the National Academy of Sciences of the United States of America 86: 1213–1217, 1989
Kelleher C, Miyauchi J, Wong G, Clark S, Minden MD, et al. Synergism between recombinant growth factors, GM-CSF and G-CSF, acting on the blast cells of acute myeloblastic leukemia. Blood 69: 1498–1503, 1987
Kelleher CA, Wong GG, Clark SC, Schendel PF, Minden MD, et al. Binding of iodinated recombinant human GM-CSF to the blast cells of acute myeloblastic leukemia. Leukemia 2: 211–215, 1988
Kharazmi A, Nielsen H, Bendtzen K. Modulation of human neutrophil and monocyte chemotaxis and Superoxide responses by recombinant TNF-alpha and GM-CSF. Immunobiology 177: 363–370, 1988
Kharazmi A, Nielsen H, Hovgaard D, Borregaard N, Nissen NI. Modulation of neutrophil and monocyte function by recombinant human granulocyte macrophage colony-stimulating factor in patients with lymphoma. European Journal of Clinical Investigation 21: 219–224, 1991
Khwaja A, Roberts PJ, Jones HM, Yong K, Jaswon MS, et al. Isoquinolinesulfonamide protein kinase inhibitors H7 and H8 enhance the effects of granulocyte-macrophage colony-stimulating factor (GM-CSF) on neutrophil function and inhibit GM-CSF receptor internalization. Blood 76: 996–1003, 1990
Kitamura T, Sato N, Arai K, Miyajima A. Expression cloning of the human IL-3 receptor cDNA reveals a shared β subunit for the human IL-3 and GM-CSF receptors. Cell 66: 1165–1174, 1991
Klausmann M, Pflüger KH, Krumwieh D, Seiler FR, Havemann K. Influence of recombinant human granulocyte-macrophage colony-stimulating factor on granulocyte functions. Behring Institute Mitteilungen 83: 265–269, 1988
Kleeberg UR, Sabersky C, Falkson G, Seeber B, Groβe M, et al. Phase-I/II study with pirarubicin (THP) and granulocyte-macrophage colony stimulating factor (rhuGM-CSF) in patients with metastatic breast cancer (MBC). Onkologie 14: 80–81, 1991
Kleinerman ES, Knowles RD, Lachman LB, Gutterman JU. Effect of recombinant granulocyte/macrophage colony-stimulating factor on human monocyte activity in vitro and following intravenous administration. Cancer Research 48: 2604–2609, 1988
Klingemann HG, Eaves AC, Barnett MJ, Reece DE, Shepherd J, et al. Recombinant GM-CSF in patients with poor graft function after bone marrow transplantation. Clinical and Investigative Medicine 13: 77–81, 1990
Kluin-Nelemans JC, Hollander AAMJ, Fibre WE, Heinhus RJ, Brand A. Leucocytoclastic vasculitis during GM-CSF therapy. British Journal of Hematology 73: 419–420, 1989
Koeffler HP, Golde DW. Cellular maturation in preleukemia. Blood 52: 355–361, 1978
Koenderman L, Warringa RAJ, Raaijmakers JAM, Bruijnzeel PLB, Kreukniet J. Induction and modulation of eosinophil chemotaxis by interleukin-3 and granulocyte-macrophage colony-stimulating factor. American Review of Respiratory Disease 143: A13, 1991
Koenigsmann M, Hiddemann W, Büchner T. Reversible leukaemic regrowth under GM-CSF treatment after chemotherapy for AML. Leukemia Research 15: 37–41, 1991
Kolenik S, Ding T-G, Longley J. Granulocyte macrophage-colony-stimulating factor (GM-CSF) decreases CD1a expression by human Langerhans cells and increases proliferation in the mixed epidermal cell-lymphocyte reaction (MELR). Journal of Investigative Dermatology 95: 359–362, 1990
Körbling M, Holle R, Haas R, Knauf W, Dörken B, et al. Autologous blood stem-cell transplantation in patients with advanced Hodgkin’s disease and prior radiation to the pelvic site. Journal of Clinical Oncology 8: 978–985, 1990
Kownatzki E, Liehl E, Aschauer H, Uhrich S. Inhibition of chemotactic migration of human neutrophilic granulocytes by recombinant human granulocyte-macrophage colony-stimulating factor. Immunopharmacology 19: 139–143, 1990
Koyanagi Y, O’Brien WA, Zhao JQ, Golde DW, Gasson JC, et al. Cytokines alter production of HIV-1 from primary mononuclear phagocytes. Science 241: 1673–1675, 1988
Krieger G, Kneba M, Vehmeyer K, Nagel GA, Weite K. Use of recombinant human granulocyte-macrophage colony stimulating factor in T-lymphoctyosis with granulocytopenia. European Journal of Haematology 44: 205–206, 1990
Kurzrock R, Talpaz M, Gomez JA, Estey EH, O’Brien S, et al. Differential dose-related haematological effects of GM-CSF in pancytopenia evidence supporting the advantage of low-over high-dose administration in selected patients. British Journal of Haematology 78: 352–358, 1991a
Kurzrock R, Talpaz M, Gutterman JU. Treatment of cyclic neutropenia with very low doses of GM-CSF. American Journal of Medicine 91: 317–318, 1991b
Kushner BH, Cheung N-KV. GM-CSF enhances 3F8 monoclonal antibody-dependent cellular Cytotoxicity against human melanoma and neuroblastoma. Blood 73: 1936–1941, 1989
Laporte JP, Fouillard L, Douay L, Eugene-Jolchine I, Isnard F, et al. GM-CSF instead of autologous bone-marrow transplantation after BEAM regimen. Lancet 338: 601–602, 1991
Lazarus HM, Andersen J, Chen MG, Variakojis D, Mansour EG, et al. Recombinant GM-CSF after autologous bone marrow transplantation for relapsed non-Hodgkin’s lymphoma: blood and bone marrow progenitor growth studies. A phase II Eastern Cooperative Oncology Group trial. Blood (in press)
Lemoli RM, Gulati SC, Strife A, Lambek C, Perez A, et al. Proliferative response of human acute myeloid leukemia cells and normal marrow enriched progenitor cells to human recombinant growth factors, IL-3, GM-CSF and G-CSF alone and in combination. Leukemia 5: 386–391, 1991
Lenarsky C, Weinberg K, Kohn D, Parkman R. Recombinant human granulocyte-macrophage colony stimulating factor (rGM-CSF) induced hematopoiesis following rejection of a histoincompatible bone marrow transplant (BMT). American Society of Hematology, 30th Annual Meeting, San Antonio, Texas December 3–6, 1988
Levine JD, Allan JD, Tessitore JH, Falcone N, Galasso F, et al. Recombinant human granulocyte-macrophage colony-stimulating factor ameliorates zidovudine-induced neutropenia in aptients with acquired immunodeficiency syndrome (AIDS)/AIDS-related complex. Blood 78: 3148–3154, 1991
Lieschke GJ, Cebon J, Morstyn G. Characterization of the clinical effects after the first dose of bacterially synthesized recombinant human granulocyte-macrophage colony-stimulating factor. Blood 74: 2534–2643, 1989a
Lieschke GJ, Maher D, Cebon J, O’Connor M, Green M, et al. Effects of bacterially synthesized recombinant human granulocyte-macrophage colony-stimulating factor in patients with advanced malignancy. Annals of Internal Medicine 110: 357–364, 1989b
Lieschke GJ, Maher D, O’Connor M, Green M, Sheridan W, et al. Phase I study of intravenously administered bacterially synthesized granulocyte-macrophage colony-stimulating factor and comparison with subcutaneous administration. Cancer Research 50: 606–614, 1990
Linch DC, Devereux S, Addison IE. The effects of recombinant human granulocyte-macrophage colony-stimulating factor on phagocyte kinetics in man. Behring Institute Mitteilungen 83: 322–323, 1988
Lindemann A, Riedel D, Oster W, Meuer SC, Dietmar B, et al. Granulocyte macrophage colony-stimulating factor induces interleukin 1 production by human polymorphonuclear neutrophils. Journal of Immunology 140: 837–839, 1988
Lindemann A, Reidel D, Oster W, Zeigler-Heitbrock HWL, Mertelsmann R, et al. Granulocyte-macrophage colony-stimulating factor induces cytokine secretion by human polymorphonuclear leukocytes. Journal of Clinical Investigation 83: 1308–1312, 1989
Lindemann A, Herrmann F, Mertelsmann R, Gamm H, Rumpelt HJ. Splenic hematopoiesis following GM-CSF therapy in a patient with hairy cell leukemia. Leukemia 4: 606–607, 1990
Link H, Freund M, Kirchner H, Stoll M, Schmid H, et al. Recombinant human granulocyte-macrophage colony stimulating factor (rhGM-CSF) after bone marrow transplantation. Behring Institute Mitteilungen 83: 313–319, 1988
Link H, Seidel J, Stoll M, Kirchner H, Linderkamp C, et al. Regeneration of granulopoiesis with recombinant human granulocyte-macrophage colony-stimulating factor after bone marrow transplantation. Haematology and Blood Transfusion 33: 741–746, 1990
Logothetis CJ, Dexeus FH, Sella A, Amato RJ, Kilbourn RG, et al. Escalated therapy for refractory urothelial tumors: methotrexate-vinblastine-doxorubicin-cisplatin plus unglycosylated recombinant human granulocyte-macrophage colony-stimulating factor. Journal of the National Cancer Institute 82: 667–672, 1990
Lopez AF, Williamson DJ, Gamble JR, Begley CG, Harlan JM, et al. A recombinant human granulocyte-macrophage colony-stimulating factor (rHGM-CSF) stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression and survival. Journal of Clinical Investigation 78: 1220–1228, 1986
Lopez AF, Lyons AB, Eglinton JM, Park LS, To LB, et al. Specific binding of human interleukin-3 and granulocyte-macrophage colony-stimulating factor to human basophils. Journal of Allergy and Clinical Immunology 85: 99–102, 1990
Lübbe AS, Schwella N, Riess H, Huhn D. Improvement of pneumonia and arthritis in Felty’s syndrome by treatment with granulocyte-macrophage colony-stimulating factor (GM-CSF). Blut 61: 379–380, 1990
Maeda K-I, Sone S, Ohmoto Y, Ogura T. A novel differentiation antigen on human monocytes that is specifically induced by granulocyte-macrophage colony-stimulating factor or IL-3. Journal of Immunology 146: 3779–3784, 1991
Man DG, Roberts BJ, Mitcheltree AC, Stiehm ER. Eosinophil cytotoxicity induced by granulocyte-macrophage colony stimulating factor (GM-CSF). Clinical Research 38: 180A, 1990
Martino P, Meloni G, Cassone A. Candidal endocarditis and treatment with fluconazole and granulocyte-macrophage colony-stimulating factor. Annals of Internal Medicine 112: 966–967, 1990
Masucci G, Wersäll P, Ragnhammar P, Mellstedt H. Granulocyte-monocyte-colony-stimulating factor augments the cytotoxic capacity of lymphocytes and monocytes in antibody-dependent cellular cytotoxicity. Cancer Immunology Immunotherapy 29: 288–292, 1989
Mayer P, Schützw E, Lam C, Kricek F, Liehl E. Recombinant murine granulocyte-macrophage colony-stimulating factor augments neutrophil recovery and enhances resistance to infections in myelosuppressed mice. Journal of Infectious Diseases 163: 584–590, 1991
Mazur EM, Cohen JL, Wong GG, Clark SC. Modest stimulatory effect of recombinant human GM-CSF on colony growth from peripheral blood human megakaryocyte progenitor cells. Experimental Hematology 15: 1128–1133, 1987
Merlin JL, Ramacci C, Weber B. Pre-clinical evaluation of human recombinant GM-CSF on human breast adeno-carcinoma cells in vitro. Abstract. European Journal of Cancer 26: 194, 1990
Metcalf D. The granulocyte-macrophage colony-stimulating factors. Science 229: 16–22, 1985
Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature 339: 27–30, 1989
Metcalf D. The colony stimulating factors. Discovery, development, and clinical applications. Cancer 65: 2185–2195, 1990
Metcalf D, Burgess AW, Johnson GR, Nicola NA, et al. In vitro actions on hemopoeitic cells of recombinant murine GM-CSF purified after production in Escherichia coli comparison with purified native GM-CSF. Journal of Cell Physiology 128: 421–431, 1986
Metcalf D, Begley CG, Williamson DJ, Nice EC, DeLarter J, et al. Hemopoietic responses in mice injected with purified recombinant murine granulocyte-macrophage colony-stimulating factor. Experimental Hematology 15: 1–9, 1987
Metcalf D, Nicola NA, Gearing DP, Gough NM. Low-affinity placenta-derived receptors for human granulocyte-macrophage colony-stimulating factor can deliver a proliferative signal to murine hemopoietic cells. Proceedings of the National Academy of Sciences of the United States of America 87: 4670–4674, 1990
McColl SR, Kreis C, DiPersio JF, Borgeat P, Naccache PH. Involvement of guanine nucleotide binding proteins in neutrophil activation and priming by GM-CSF. Blood 73: 588–591, 1989
McColl SR, Beauseigle D, Gilbert C, Naccache PH. Priming of the human neutrophil respiratory burst by granulocyte-macrophage colony-stimulating factor and tumor necrosis factor-α involves regulation at a post-cell surface receptor level. Enhancement of the effect of agents which directly activate G proteins. Journal of Immunology 145: 3047–3053, 1990a
McColl SR, Krump E, McDonald PP, Braquet M, Naccache PH, et al. Enhancement of platelet-activating factor-induced leukotriene synthesis in neutrophils by granulocyte-macrophage colony-stimulating factor (GM-CSF): studies on the mechanism of action of GM-CSF. Journal of Lipid Mediators 2: S119–S127, 1990b
McNiece I, Andrews R, Stewart M, Clark S, Boone T, et al. Action of interleukin-3, G-CSF, and GM-CSF on highly enriched human hematopoietic progenitor cells: synergistic interaction of GM-CSF plus G-CSF. Blood 74: 110–114, 1989
McNiece IK, Langley KE, Zsebo KM. Recombinant human stem cell factor synergises with GM-CSF, G-CSF, IL-3 and Epo to stimulate human progenitor cells of the myeloid and erythroid lineages. Experimental Hematology 19: 226–231, 1991
Michon J, Andreu G, Boccacio C, Lopez M, Mortel O, et al. Recombinant human GM-CSF prior to peripheral blood stem cell harvest for transplantation. A report of a paediatric case. International Symposium on Peripheral Blood Stem Cell Autografts, Mulhouse, France, October 15–17, 1989
Migliaccio AR, Bruno M, Migliaccio G. Evidence for direct action of human biosynthetic (recombinant) GM-CSF on erythroid progenitors in serum-free culture. Blood 70: 1867–1871, 1987
Migliaccio G, Migliaccio AR, Adamson JW. In vitro differentiation of human granulocyte/macrophage and erythroid progenitors: comparative analysis of the influence of recombinant human erythropoietin, G-CSF, GM-CSF, and IL-3 in serumsupplemented and serum-deprived cultures. Blood 72: 248–256, 1988
Milliken S, Powles R, Smith C, Tiley C. The use of recombinant GM-CSF after allogeneic bone marrow transplantation — a randomised double-blind trial. Australia and New Zealand Journal of Medicine 21 (Suppl.): 159, 1991
Mitsuyasu R, Levine J, Miles SA, DeLeo M, Oette D, et al. Effects of long term subcutaneous (SC) administration of recombinant granulocyte-macrophage colony stimulating factor (GM-CSF) in patients with HIV-related leukopenia. American Society of Hematology, Texas, December 3–6: 66, 1988
Molloy RG, Nestor M, Mannick J, Rodrick M. GM-CSF: a therapeutic role in the prevention of mortality after burn sepsis. Abstract 7539.FASEB Journal 5: A1669, 1991
Monroy RL, Skelly RR, Taylor P, Dubois A, Donahue RE, et al. Recovery from severe hematopoietic suppression using recombinant human granulocyte-macrophage colony-stimulating factor. Experimental Hematology 16: 344–348, 1988
Morrissey PJ, Grabstein KH, Reed SG, Conlon PJ. Granulocyte/macrophage colony stimulating factor. A potent activation signal for mature macrophages and monocytes. International Archives of Allergy and Applied Immunology 88: 40–45, 1989
Motoji T, Takanashi M, Fuchinoue M, Masuda M, Oshimi K, et al. Effect of recombinant GM-CSF and recombinant G-CSF on colony formation of blast progenitors in acute myeloblastic leukemia. Experimental Hematology 17: 56–60, 1989
Motoji T, Takanashi M, Masuda M, Nakayama K, Oshimi K, et al. Colony stimulating activities of GM-CSF, G-CSF and IL-3 on blast progenitors from acute myeloblastic leukemia. Leukemia and Lymphoma 2: 407–414, 1990
Mufti GJ, Dalton DAG. Myelodysplastic syndromes: natural history and features of prognostic importance. Clinical Haematology 15: 953–971, 1986
Muschenich M, Burdach St, Josephs W, Frisch J, Schulz G, et al. Granulocyte/macrophage colony stimulating factor shortens duration of neutropenia and infections following aplasiogenic chemotherapy in children and adolescents with solid tumors. Abstract. Medical and Pediatric Oncology 18: 517, 1990
Naccache PH, Faucher N, Borgeat P, Gasson JC, DiPersio JF. Granulocyte-macrophage colony-stimulating factor modulates the excitation-response coupling sequence in human neutrophils. Journal of Immunology 140: 3541–3546, 1988
Nachbaur D, Denz H, Zwierzina H, Schmalzl F, Huber H. Stimulation of colony formation of various human carcinoma cell lines by rhGm-CSF and rhIL-3. Cancer Letters 50: 197–201, 1990
Naeim F, Champlin R, Nimer S. Bone marrow changes in patients with refractory aplastic anemia treated by recombinant GM-CSF. Hematologic Pathology 4: 79–85, 1990
Nagler A, Ginzton N, Negrin R, Bang D, Donlon T, et al. Effects of recombinant human granulocyte colony stimulating factor and granulocyte-monocyte colony stimulating factor on in vitro hemopoiesis in the myelodysplastic syndromes. Leukemia 4: 193–202, 1990
Namiki M, Hara H. Enhancement of colony-forming activity of granulocyte-macrophage colony-stimulating factor by monocytes in vitro. Blood 74: 918–924, 1989
Neidhart J, Stidley C, Mangalik A, Sarmiento L, Oette D, et al. Optimum dosing of GM-CSF for dose intensive chemotherapy with cyclophosphamide, VP-16, and cisplatinum. Abstract 1153. Proceedings of the American Association for Cancer Research 32: 193, 1991.
Nemunaitis J, Singer JW, Buckner CD, Hill R, Storb R, et al. Use of recombinant human granulocyte-macrophage colony-stimulating factor in autologous marrow transplantation for lymphoid malignancies. Blood 72: 834–836, 1988
Nemunaitis J, Singer JW, Buckner CD, Hill R, Storb R, et al. Use of human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) in autologous transplantation for lymphoid malignancies. Autologous Bone Marrow Transplantation: Proceedings of the Third International Symposium, pp. 631–636, Houston, 1989
Nemunaitis J, Singer JW, Buckner CD, Durnam D, Epstein C, et al. Use of recombinant human granulocyte-macrophage colony-stimulating factor in graft failure after bone marrow transplantation. Blood 76: 245–253, 1990
Nemunaitis J, Buckner CD, Appelbaum FR, Higano CS, Mori M, et al. Phase I/II trial of recombinant human granulocyte-macrophage colony-stimulating factor following allogenic bone marrow transplantation. Blood 77: 2065–2071, 1991a
Nemunaitis J, Rabinowe SN, Singer JW, Bierman PJ, Vose JM, et al. Recombinant granulocyte-macrophage colony-stimulating factor after autologous bone marrow transplantation for lymphoid cancer. New England Journal of Medicine 324: 1773–1778, 1991b
Nemunaitis J, Singer JW, Buckner CD, Mori T, Laponi J, et al. Long-term follow-up of patients who received recombinant human granulocyte-macrophage colony stimulating factor after autologous bone marrow transplantation for lymphoid malignancy. Bone Marrow Transplantation 7: 49–52, 1991c
Neuman E, Huleatt JW, Jack RM. Granulocyte-macrophage colony-stimulating factor increases synthesis and expression of CR1 and CR3 by human peripheral blood neutrophils. Journal of Immunology 145: 3325–3332, 1990
Nissen C, Tichelli A, Gratwohl A, Speck B, Milne A, et al. Failure of recombinant human granulocyte-macrophage colony-stimulating factor therapy in aplastic anemia patients with very severe neutropenia. Blood 72: 2045–2047, 1988
Oksenberg D, Dieckmann BS, Greenberg PL. Functional interactions between colony-stimulating factors and the insulin family hormones for human myeloid leukemic cells. Cancer Research 50: 6471–6477, 1990
Onetto-Pothier N, Aumont N, Haman A, Bigras C, Wong GG, et al. Characterization of granulocyte-macrophage colony-stimulating factor receptor on the blast cells of acute myeloblastic leukemia. Blood 75: 59–66, 1990
Oster W, Lindemann A, Mertelsmann R, Herrmann F. Granulocyte-macrophage colony-stimulating factor (CSF) and multilineage CSF recruit monocytes to express granulocyte CSF. Blood 73: 64–67, 1989
Owen Jr WF, Rothenberg ME, Silberstein DS, Gasson JC, Stevens RL, et al. Regulation of human eosinophil viability, density, and function by granulocyte/macrophage colony-stimulating factor in the presence of 3T3 fibroblasts. Journal of Experimental Medicine 166: 129–141, 1987
Palmblad J, Jonson B, Kanerud L. Treatment of drug-induced agranulocytosis with recombinant GM-CSF. Journal of Internal Medicine 228: 537–539, 1990
Paolucci P, Calzolari P, Giovannini M, Prete A, Andreani G, et al. rhGM-CSF and growth of short term culture neuroblastoma. Abstract no. 55. SIOP XXIII Meeting, pp. 352, 1991
Park LS, Friend D, Price V, Anderson D, Singer J, et al. Heterogeneity in human interleukin-3 receptors. A subclass that binds human granulocyte/macrophage colony stimulating factor. Journal of Biological Chemistry 264: 5420–5427, 1989
Pavelic K, Baltic V, Spaventi S. Artificial reversion of acute myeloid leukemia cells into normal phenotype. International Journal of Biochemistry 22: 553–538, 1990
Pébusque M-J, Lopez M, Torres H, Carotti A, Guilbert L, et al. Growth response of human myeloid leukemia cells to colony-stimulating factors. Experimental Hematology 16: 360–366, 1988
Pébusque M-J, Fay C, Lafage M, Sempéré C, Saeland S, et al. Recombinant human IL-3 and G-CSF act synergistically in stimulating the growth of acute myeloid leukemia cells. Leukemia 3: 200–205, 1989
Perkins RC, Scheule RK, Vadhan SK, Holian A. The effect of in vivo GM-CSF therapy on human monocyte function. Abstract. American Review of Respiratory Disease 143: A233, 1991
Perno C-F, Yarchoan R, Cooney DA, Harman NR, Webb DSA, et al. Replication of human immunodeficiency virus in monocytes. Granulocyte/macrophage colony-stimulating factor (GM-CSF) potentiates viral production yet enhances the antiviral effect mediated by 3’-azido-2’3’-dideoxythymidine (AZT) and other dideoxynucleoside congeners of thymidine. Journal of Experimental Medicine 169: 933–951, 1989
Peters WP, Kurtzberg J, Atwater S, Borowtiz M, Rao M, et al. The use of recombinant human granulocyte-macrophage colony-stimulating factor in autologous bone marrow transplantation. Hematopoietic Growth Factors in Transfusion Medicine: 121–128, 1990
Peters WP, Shogan J, Shpall EJ, Jones RB, Kim CS. Recombinant human granulocyte-macrophage colony-stimulating factor produces fever. Lancet 8591: 950, 1988
Phillips N, Jacobs S, Stoller R, Earle M, Przepiorka D, et al. Effect of recombinant human granulocyte-macrophage colony-stimulating factor on myelopoiesis in patients with refractory metastatic carcinoma. Blood 74: 26–34, 1989
Piao YF, Tojo A, Urabe A, Takaku F, Suzuki T, et al. Effects of recombinant human G- and GM-CSF on the proliferation of leukemic blast progenitors from acute myeloblastic leukemia patients. Acta Haematologica Japonica 51: 1115–1121, 1988
Pluda JM, Yarchoan R, Smith PD, McAtee N, Shay LE, et al. Subcutaneous recombinant granulocyte-macrophage colony-stimulating factor used as a single agent and in an alternating regimen with azidothymidine in leukopenic patients with severe human immunodeficiency virus infection. Blood 76: 463–472, 1990
Potter MN, Mott MG, Oakhill A. The successful treatment of a case of very severe aplastic anaemia with granulocyte-macrophage colony stimulating factor and anti-lymphocyte globulin. British Journal of Haematology 75: 618, 1990
Poubelle PE, Bourgoin S, Naccache PH, Borgeat P. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and opsonization synergistically enhance leukotriene B4(LTB4) synthesis induced by phagocytosis in human neutrophils. Agents and Actions 27: 3–4, 1989
Reed SG, Nathan CF, Pihl DL, Rodricks P, Shanebeck K, et al. Recombinant granulocyte-macrophage colony-stimulating factor activates macrophages to inhibit Trypsanoma cruzii and release hydrogen peroxide. Journal of Experimental Medicine 166: 1734–1746, 1987
Richter J, Andersson T, Olsson I. Effect of tumor necrosis factor and granulocyte/macrophage colony-stimulating factor on neutrophil degranulation. Journal of Immunology 142: 3199–3205, 1989
Rifkin RM, Hersh EM, Salmon SE. A phase I study of therapy with recombinant granulocyte-macrophage colony-stimulating factor administered by IV bolus or continuous infusion. Behring Institute Mitteilungen 83: 125–133, 1988
Riklis I, Kletter Y, Bleiberg I, Fabian I. Biological properties in vitro of a combination of recombinant murine interleukin-3 and granulocyte-macrophage colony-stimulating factor. European Journal of Haematology 42: 375–381, 1989
Robinson BE, McGrath HE, Quesenberry PJ. Recombinant murine granulocyte macrophage colony-stimulating factor has megakaryocyte colony-stimulating activity and augments megakaryocyte colony stimulation by interleukin 3. Journal of Clinical Investigation 79: 1648–1652, 1987
Roilides E, Mertins S, Eddy J, Walsh TJ, Pizzo PA, et al. Impairment of neutrophil chemotactic and bactericidal function in children infected with human immunodeficiency virus type 1 and partial reversal after in vitro exposure to granulocyte-macrophage colony-stimulating factor. Journal of Pediatrics 117: 531–540, 1990
Rosenfeld CS, Sulecki M, Evans C, Shadduck RK. Comparison of intravenous versus subcutaneous recombinant human granulocyte-macrophage colony-stimulating factor in patients with primary myelodysplasia. Experimental Hematology 19: 273–277, 1991
Russo CL, Glader BE, Israel RJ, Galasso F. Treatment of neutropenia associated with dyskeratosis congenita with granulocyte-macrophage colony-stimulating factor. Lancet 336: 751–752, 1990
Rusthoven J, Eisenhauer LLE, Mazurka CJ. A phase I study of GM-CSF cyclophosphamide (CP) and escalating doses of carboplatin (CBDCA) in chemotherapy-naive patients with ovarian cancer. Gynecologic Oncology 40: 169–170, 1991
Saeland S, Caux C, Favre C, Duvert V, Pébusque M-J, et al. Combined and sequential effects of human IL-3 and GM-CSF on the proliferation of CD344 hematopoietic cells from cord blood. Blood 73: 1195–1201, 1989
Salmon SE, Lui R. Effects of granulocyte-macrophage colony-stimulating factor on the in vitro growth of human solid tumors. Journal of Clinical Oncology 7: 1346–1350, 1989
Sasaki H, Koiso Y, Ikuta K, Kajigaya Y, Funabiki T, et al. Three quantitative assays for human erythroid burst-promoting activity of recombinant growth factors and of omentum-conditioned medium. Experimental Hematology 18: 84–88, 1990
Scadden DT. Granulocyte macrophage colony stimulating factor (GM-CSF) in AIDS. Hematopoietic Growth Factors in Transfusion Medicine: 163–176, 1990
Scadden DT, Bering HA, Levine J, Bresnahan J, Evans L, et al. GM-CSF as an alternative to dose modification of the combination zidovudine and interferon-α in the treatment of AIDS-associated Kaposi’s sarcoma. American Journal of Clinical Oncology: S40–S44, 1991
Schroten H, Wendel U, Burdach S, Roesler J, Breidenbach T, et al. Colony-stimulating factors for neutropenia in glycogen storage disease Ib. Lancet 337: 736–737, 1991
Schuening FG, Storb R, Goehle S, Nash R, Graham TC, et al. Stimulation of canine hematopoiesis by recombinant human granulocyte-macrophage colony-stimulating factor. Experimental Hematology 17: 889–894, 1989
Schuster MW, Thompson JA, Larson R, Allen SL, O’Laughlin R, et al. Randomized trial of subcutaneous granulocyte-macrophage colony-stimulating factor (GM-CSF) versus observation in patients with myelodysplastic syndrome. Journal of Cancer Research and Clinical Oncology 116 (Suppl.): 1079, 1990
Shadduck RK, Waheed A, Evans C, Sulecki C, Rosenfeld CS. Serum and urinary levels of recombinant human granulocyte-macrophage colony-stimulating factor: assessment after intravenous infusion and subcutaneous injection. Experimental Hematology 18: 601, 1990
Sieff CA. Hematopoietic growth factors. Journal of Clinical Investigation 79: 1549–1557, 1987
Silberstein DS, Owen WF, Gasson JC, DiPersio JF, Bolde DW, et al. Enhancement of human eosinophil cytotoxicity and leukotriene synthesis by biosynthetic (recombinant) granulocyte-macrophage colony-stimulating factor. Journal of Immunology 137: 3290–3294, 1986
Smith T, Grossberg H. Successful use of granulocyte-macrophage colony-stimulating factor in patients with acute lymphocytic leukemia. American Journal of Medicine 89: 384–386, 1990
Smith PD, Lamerson CL, Banks SM, Saini SS, Wahl LM, et al. Granulocyte-macrophage colony-stimulating factor augments human monocyte fungicidal activity for Candida albicans. Journal of Infectious Diseases 161: 999–1005, 1990a
Smith PD, Lamerson CL, Wong HL, Wahl LM, Wahl, SM. Granulocyte-macrophage colony-stimulating factor stimulates human monocyte accessory cell function. Journal of Immunology 144: 3829–3834, 1990b
Smith RJ, Justen JM, Sam LM. Recombinant human granulocyte-macrophage colony-stimulating factor induces granule exocytosis from human polymorphonuclear neutrophils. Inflammation 14: 83–92, 1990c
Socinski MA, Elias A, Schnipper L, Cannistra SA, Antman KH, et al. Granulocyte-macrophage colony stimulating factor expands the circulating haemopoietic progenitor cell compartment in man. Lancet 8596: 1194–1198, 1988a
Socinksi MA, Cannistra SA, Sullivan R, Elias A, Antman K, et al. Granulocyte-macrophage colony-stimulating factor induces the expression of the CD11b surface adhesion molecule on human granulocytes in vivo. Blood 72: 691–697, 1988b
Sorensen PHB, Mui AL-F, Murthy SC, Krystal G. Interleukin-3, GM-CSF, and TPA induce distinct phosphorylation events in an interleukin 3-dependent multipotential cell line. Blood 73: 406–418, 1989
Stehle B, Weiss C, Ho AD, Hunstein W. Serum levels of tumor necrosis factor α in patients treated with granulocyte-macrophage colony-stimulating factor. Blood 75: 1895–1899, 1990
Steis RG, VanderMolen LA, Longo DL, Clark JW, Smith JW, et al. Recombinant human granulocyte-macrophage colony-stimulating factor in patients with advanced malignancy: a phase Ib trial. Journal of the National Cancer Institute 82: 697–703, 1990
Steward WP, Scarffe JH, Austin R, Bonnern E, Thatcher N, et al. Recombinant human granulocyte macrophage colony stimulating factor (rhGM-CSF) given as daily short infusions — a phase I dose-toxicity study. British Journal of Cancer 59: 142–145, 1989
Steward WP, Scarffe JH, Dirix LY, Chang J, Radford JA, et al. Granulocyte-macrophage colony stimulating factor (GM-CSF) after high-dose melphalan in patients with advanced colon cancer. British Journal of Cancer 61: 749–754, 1990
Steward WP, Verweij J, Somers R, Blackledge G, Clavel M, et al. High dose chemotherapy with two schedules of recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) in the treatment of advanced adult soft tissue sarcomas. Abstract 1240. Proceedings of the American Society of Clinical Oncology: 27th Annual Meeting, p. 349, Houston, 10 March, 1991
Stute N, Huhn RD, Furman WL, Evans WE. Pharmacokinetics of IV recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF) in children with cancer. Clinical Pharmacology and Therapeutics 49: 197, 1991
Sulecki M, Rosenfeld CS, Przepiorka D, Bloom EJ, Buhles Jr W, et al. Treatment of ganciclovir induced neutropenia with recombinant human GM-CSF. American Journal of Medicine 90: 401–402, 1991
Sullivan R, Griffin J, Wright J, Melnick DA, Leavitt JL, et al. Effects of recombinant human granulocyte-macrophage colony-stimulating factor on intracellular pH in mature granulocytes. Blood 72: 1665–1673, 1988
Sullivan R, Fredette JP, Griffin J, Leavitt JL, Simons ER, et al. An elevation in the concentration of free cytosolic calcium is sufficient to activate the oxidative burst of granulocytes primed with recombinant human granulocyte-macrophage colony-stimulating factor. Journal of Biological Chemistry 264: 6302–6309, 1989
Suttorp M, Schmitz N, Prange E, Ganser A, Löffler H, et al. Successful stimulation of autologous bone marrow recovery by GM-CSF and IL-3 after unrelated donor BMT for juvenile CML complicated by graft failure. Bone Marrow Transplantation 7 (Suppl. 2): 84, 1991
Suzuki K, Yamamoto T, Murayama T, Kuze F. Granulocyte-macrophage colony-stimulating factor and tumor necrosis factor alpha can activate human alveolar macrophages to inhibit growth of mycobacterium avium complex (MAC). American Review of Respiratory Disease 143: A808, 1991
Tai PC, Spry CJ. The effects of recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-3 on the secretory capacity of human blood eosinophils. Clinical and Experimental Immunology 80: 426–434, 1990
Tanaka T, Okamura S, Okada K, Suga A, Shimono N, et al. Protective effect of recombinant murine granulocyte-macrophage colony-stimulating factor against Pseudomonas aeruginosa infection in leukocytopenic mice. Infection and Immunity 5: 1792–1799, 1989
Tanikawa S, Nakao I, Tsuneoka K, Nara N. Effects of recombinant granulocyte colony-stimulating factor (rG-CSF) and recombinant granulocyte-macrophage colony-stimulating factor (rGM-CSF) on acute radiation hematopoietic injury in mice. Experimental Hematology 17: 883–888, 1989
Tarella C, Ferrero D, Bregni M, Siena S, Gallo E, et al. Peripheral blood expansion of early progenitor cells after high-dose cyclophosphamide and rhGM-CSF. European Journal of Cancer 27: 22–27, 1991
Tawhid H, Labastide W, Barker C, Rees J. Cooperative effects of human recombinant granulocyte-macrophage colony stimulating factor and human recombinant erythropoietin in inducing erythroid differentiation of the human erythroleukaemia cell line K 562 clonogenic cells. Leukemia 13: 127–130, 1989
Thomas S, Clark SC, Rappeport JM, Nathan DG, Emerson SG. Deficient T cell granulocyte-macrophage colony stimulating factor production in allogeneic bone marrow transplant recipients. Transplantation 49: 703–708, 1990
Thomassen MJ, Barna BP, Rankin D, Wiedemann HP, Ahmad M. Differential effect of recombinant granulocyte macrophage colony-stimulating factor on human monocytes and alveolar macrophages. Cancer Research 49: 4086–4089, 1989
Thomassen MJ, Rankin D, Barna BP, Meeker DP, Wiedemann HP, et al. Mechanism of granulocyte macrophage colony stimulating factor (GM-CSF) induced tumoricidal activity. Abstract. America Review of Respiratory Disease 141: 1746, 1990
Thomassen MJ, Ahmad M, Barna BP, Antal J, Wiedemann HP, et al. Induction of cytokine messenger RNA and secretion in alveolar macrophages and blood monocytes from patients with lung cancer receiving granulocyte-macrophage colony-stimulating factor therapy. Cancer Research 51: 857–962, 1991
Thomssen C, Nissen C, Gratwohl A, Tichelli A, Stern A. Agranulocytosis associated with T-gamma-lymphocytosis: no improvement of peripheral blood granulocyte count with human-recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF). British Journal of Haematology 71: 157–158, 1989
Tirelli U, Sorio R, Monfardini S, Lazzarin A, Rossi G, et al. Recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) in patients with AIDS-related tumors and chemotherapy-induced bone-marrow suppression. Correspondence. Journal of Acquired Immune Deficiency Syndromes 3: 1115, 1990
To LB, Dyson PG, Juttner CA. Cell-dose effect in circulating stem-cell autografting. Lancet 2: 404–405, 1986
Tohyama K, Ohmori S, Michishita M, Ueda T, Ueda Y, et al. Effects of recombinant G-CSF and GM-CSF on in vitro differentiation of the blast cells of RAEB and RAEB-T. European Journal of Haematology 42: 348–353, 1989
Tomioka K, Bochner BS, Derse-Anthony C, Lichtenstein LM, Schleimer RP. GM-CSF enhances PAF- and FMLP-induced eosinophil expression of adherence molecules (CD11b) and increases FMLP-induced adherence to endothelial cells. Journal of Allergy and Clinical Immunology 87: 654, 1991
Twentyman PR, Wright KA. Failure of GM-CSF to influence the growth of small cell and non-small cell lung cancer cell lines in vitro. European Journal of Cancer 27: 6–8, 1991
Vadhan-Raj S, Keating M, LeMaistre A, Hittelman WN, McCredie K, et al. Effects of recombinant human granulocyte-macrophage colony-stimulating factor in patients with myelodysplastic syndromes. New England Journal of Medicine 317: 1545–1552, 1987
Vadhan-Raj S, Buescher S, Broxmeyer HE, LeMaistre A, Lepe-Zuniga JL, et al. Stimulation of myelopoiesis in patients with aplastic anemia by recombinant human granulocyte-macrophage colony-stimulating factor. New England Journal of Medicine 319: 1628–1634, 1988a
Vadhan-Raj S, Buescher S, LeMaistre A, Keating M, Walters R, et al. Stimulation of hematopoiesis in patients with bone marrow failure and in patients with malignancy by recombinant human granulocyte-macrophage colony-stimulating factor. Blood 72: 134–141, 1988b
Vadhan-Raj S, Hittelman WN, Ventura C, Buescher S, Keating MJ, et al. Granulocyte-macrophage colony-stimulating factor and myelodysplastic syndromes. New England Journal of Medicine 319: 51–53, 1988c
Vadhan-Raj S, Broxmeyer HE, Spitzer G, LeMaistre A, Hultman S, et al. Stimulation of nonclonal hematopoiesis and suppression of the neoplastic clone after treatment with recombinant human granulocyte-macrophage colony-stimulating factor in a patient with therapy-related myelodysplastic syndrome. Blood 74: 1491–1498, 1989
Vadhan-Raj S, Jeha SS, Buescher S, LeMaistre A, Yee G, et al. Stimulation of myelopoiesis in a patient with congenital neutropenia: biology and nature of response to recombinant human granulocyte-macrophage colony-stimulating factor. Blood 75: 858–864, 1990
Vadhan-Raj S, Broxmeyer HE, Hittleman WN, Papadopoulos NE, Plager C, et al. Abrogating chemotherapy-induced myelosuppression by GM-CSF: optimizing the schedule. Abstract 1241. Proceedings of the American Society of Clinical Oncology: 27th Annual Meeting, pp. 349, Houston, March 10, 1991
Vallance SJ, Downes CP, Cragoe EJ, Whetton AD. Granulocyte-macrophage colony-stimulating factor can stimulate macrophage proliferation via persistent activation of NA+/H+ anti-port. Evidence for two distinct roles for NA+/H+ antiport activation. Biochemical Journal 265: 359–364, 1990
Vellenga E, Deiwel HR, Touw IP, Löwenberg B. Patterns of acute myeloid leukemia colony growth in response to recombinant granulocyte-macrophage colony-stimulating factor (rGM-CSF). Experimental Hematology 15: 652–656, 1987a
Vellenga E, Ostapovicz D, ORourke B, Griffin J. Effects of recombinant IL-3 GM-CSF, and G-CSF on proliferation of leukemic clonogenic cells in short-term and long-term cultures. Leukemia 1: 584–589, 1987b
Ventura GJ, Reading CL, Hester JP, Vadhan-Raj S. Circulating myeloid progenitor cell kinetics during hematologic recovery from chemotherapy and subsequent recombinant human granulocyte-macrophage colony-stimulating factor administration. Acta Haematologica 84: 175–181, 1990
Villeval J-L, Duhrsen U, Morstyn G, Metcalf D. Effect of recombinant human granulocyte-macrophage colony-stimulating factor on progenitor cells in patients with advanced malignancies. British Journal of Haematology 74: 36–44, 1990
Visani G, Gamberi B, Greenberg P, Advani R, Gulati S, et al. The use of GM-CSF as an adjunct to autologous/syngeneic bone marrow transplantation: a prospective randomized controlled trial. Bone Marrow Transplantation 7 (Suppl 2): 81, 1991
Visani G, Tosi P, Gamberi B, Cenacchi A, Mazzanti P, et al. Accelerated hemopoietic recovery after chemotherapy and autologous bone marrow transplantation in hematological malignancies using recombinant GM-CSF preliminary results obtained in 14 cases. Haematologica 75: 551–554, 1990
Vose JM, Bierman PJ, Kessinger A, Coccia PF, Anderson J, et al. The use of recombinant human granulocyte-macrophage colony stimulating factor for the treatment of delayed engraftment following high dose therapy and autologous hematopoietic stem cell transplantation for lymphoid malignancies. Bone Marrow Transplantation 7: 139–143, 1991
Waksman Y, Golde DW, Savion N, Fabian I. Granulocyte-macrophage colony-stimulating factor enhances cationic antimicrobial protein synthesis by human neutrophils. Journal of Immunology 144: 3437–3443, 1990
Walker F, Nicola NA, Metcalf D, Burgess AW. Hierarchical down-modulation of hemopoietic growth factor receptors. Cell 43: 269–276, 1985
Wang JM, Colella S, Allavena P, Mantovani A. Chemotactic activity of human recombinant granulocyte-macrophage colony-stimulating factor. Immunology 60: 439–444, 1987
Wang M, Friedman H, Djeu JY. Enhancement of human monocyte function against Candida albicans by the colony-stimulating factors (CSF): IL-3, granulocyte-macrophage-CSF, and macrophage-CSF. Journal of Immunology 143: 671–677, 1989
Ward CJ, Crocker J, Chan SJ, Stockley RA, Burnett D. Changes in the expression of elastase and cathepsin B with differentiation of U937 promonocytes by GMCSF. Biochemical and Biophysical Research Communications 167: 659–664, 1990
Weiser WY, Van Niel CF, Clark SC, David JR, Remold HG. Recombinant human granulocyte-macrophage colony-stimulating factor activates intracellular killing of Leishmania donovani by human monocyte-derived macrophages. Journal of Experimental Medicine 166: 1436–1446, 1987
Weite K, Zeidler C, Reiter A, Müller W, Odenwald E, et al. Differential effects of granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor in children with severe congenital neutropenia. Blood 75: 1056–1063, 1990
Williams DE, Straneva JE, Cooper S, Shadduck RK, Waheed A, et al. Interactions between purified murine colony-stimulating factors (natural CSF-1, recombinant GM-CSF, and recombinant IL-3) on the in vitro proliferation of purified murine granulocyte-macrophage progenitor cells. Experimental Hematology 15: 1007–1012, 1987
Williams GT, Smith CA, Spooncer E, Dexter TM, Taylor DR. Haemopoietic colony stimulating factors promote cell survival by suppressing apoptosis. Nature 343: 76–79, 1990
Wing EJ, Magee DM, Whiteside TL, Kaplan SS, Shadduck RK. Recombinant human granulocyte/macrophage colony-stimulating factor enhances monocyte cytotoxicity and secretion of tumor necrosis factor α and interferon in cancer patients. Blood 73: 643–646, 1989
Wirthmueller U, De Weck AL, Dahinden CA. Platelet-activating factor production in human neutrophils by sequential stimulation with granulocyte-macrophage colony-stimulating factor and the chemotactic factors C5A or formyl-methionyl-leucyl-phenylalanine. Journal of Immunology 142: 3213–3218, 1989
Wodzinski MA, Hampton KK, Reilly JT. Differential effect of G-CSF and GM-CSF in acquired chronic neutropenia. British Journal of Haematology 77: 249, 1991
Yamada H, Tubaki K, Ashida T, Urase F, Orita T, et al. Does recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) play a crucial role in the pathogenesis of atopic dermatitis after bone marrow transplantation (BMT)? Medical Science Research 19: 395, 1991
Yamaga S, Okamura S, Otsuka T, Niho Y. Effect of granulocyte-macrophage colony-stimulating factor on chemiluminescence of human neutrophils. International Journal of Cell Cloning 7: 50–58, 1989
Yuo A, Kitagawa S, Ohsaka A, Saito M, Takaku F. Stimulation and priming of human neutrophils by granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor: qualitative and quantitative differences. Biochemical and Biophysical Research Communications 171: 491–497, 1990
Zimmerli W, Zarth A, Gratwohl A, Nissen C, Speck B. Granulocyte-macrophage colony-stimulating factor for granulocyte defects of bone marrow transplant patients. Correspondence. Lancet: 494, 1989
Zuckerman SH, Surprenant YM. Induction of endothelial cell/macrophage procoagulant activity: synergistic stimulation by gamma interferon and granulocyte-macrophage colony stimulating factor. Thrombosis and Haemostasis 61: 178–182, 1989
Zuckermann SH, Surprenant YM, Tang J. Synergistic effect of granulocyte-macrophage colony-stimulating factor and 1,25 dihydroxyvitamin D3 on the differentiation of the human monocytic cell line U937. Blood 71: 619–624, 1988
Author information
Authors and Affiliations
Additional information
Various sections of the manuscript reviewed by: J.H. Antin, Hematology Division, Brigham and Women’s Hospital Boston, Massachusetts, USA; H.E. Broxmeyer, School of Medicine, Indiana University, Indianapolis, Indiana USA; S.W. Edwards, Department of Biochemistry, The University of Liverpool, Liverpool, England; A. Ganser, Department of Hematology, University of Frankfurt, Frankfurt, Federal Republic of Germany; A.M. Gianni, Divisione di Oncologia Medica, Istituto Nationale per lo Studio e la Cura dei Tumori, Milano, Italy; A.D. Ho, Faculty of Medicine, University of Ottawa, Ontario, Canada; A.V. Hoffbrand, Department of Haematology, The Royal Free Hampstead NHS Trust, London, England; D. Linch, Department of Haematology, University College and Middlesex School of Medicine, London, England; D. Metcalf, Cancer Research Unit, The Walter and Eliza Hall Institute of Medical Research, Melbourne,Victoria, Australia;J.J Nemunaitis, Hematopoiesis Program, Western Pennsylvania Cancer Institute, Pittsburgh, Pennsylvania, USA; S. Okamura, Cancer Center, Kyushu University Hospital, Fukuoka, Japan; W.P. Steward, Beaston Oncology Centre, Western Infirmary Glasgow, Scotland; S. Vadhan-Raj, Department of Clinical Immunology and Biological Therapy, MD Anderson Cancer Center, The University of Texas, Houston, Texas, USA.
Rights and permissions
About this article
Cite this article
Grant, S.M., Heel, R.C. Recombinant Granulocyte-Macrophage Colony-Stimulating Factor (rGM-CSF). Drugs 43, 516–560 (1992). https://doi.org/10.2165/00003495-199243040-00008
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199243040-00008