Abstract
Objective: To characterize levetiracetam pharmacokinetics, identify significant covariate relationships and identify doses in children that achieve blood concentrations similar to those observed in adults.
Methods: Nonlinear mixed-effects modelling was used to analyse pooled data collected from 228 children with epilepsy aged 3 months to 18 years in five trials of adjunctive levetiracetam therapy. Simulations were used to identify dosing regimens achieving levetiracetam steady-state peak and trough plasma concentrations similar to those attained in adults receiving the recommended starting dose for adjunctive therapy (500 mg twice daily). The covariates considered for inclusion in the base model were age, bodyweight, gender, race, body surface area (BSA), body mass index (BMI), creatinine clearance (CLCR), levetiracetam dose, concomitant antiepileptic drug (AED) by category (neutral, enzyme inducer, inhibitor, combination of inducer and inhibitor), and benzodiazepines.
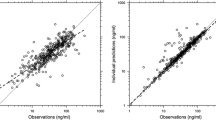
Results: A one-compartment model with first-order absorption and elimination best characterized the data. The following significant covariates were identified: (i) age on the absorption rate constant (ka); (ii) bodyweight, dose, CLCR and concomitant enzyme-inducing AED on plasma oral clearance (CL/F); and (iii) bodyweight on the apparent volume of distribution after oral administration (Vd/F). The main explanatory covariates were age on ka, bodyweight on CL/F and Vd/F, and enzyme-inducing AED on CL/F, of which bodyweight was the most influential covariate. Dosing can be carried out with either 10 mg/kg of oral solution twice daily in children weighing <50 kg and a 500-mg tablet twice daily in those weighing >50 kg or, when patients favour a solid formulation, 10 mg/kg of oral solution twice daily in children weighing <20 kg, a 250-mg tablet twice daily in those weighing 20–40 kg, and a 500-mg tablet twice daily in those weighing >40 kg. All of these doses achieved steady-state peak and trough plasma concentrations similar to those observed in adults following the recommended starting dose for adjunctive therapy (500 mg twice daily).
Conclusions: The most influential covariate of levetiracetam pharmacokinetics in children is bodyweight. A starting dose of levetiracetam 10 mg/kg twice daily ensures the same exposure in children as does 500 mg twice daily in adults.






Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Ben-Menachem E, Falter U. Efficacy and tolerability of levetiracetam 3000 mg/d in patients with refractory partial seizures: a multicenter, double-blind, responder-selected study evaluating monotherapy. European Levetiracetam Study Group. Epilepsia 2000 Oct; 41(10): 1276–83
Cereghino JJ, Biton V, Abou-Khalil B, et al. Levetiracetam for partial seizures: results of a double-blind, randomized clinical trial. Neurology 2000 Jul 25; 55(2): 236–42
Shorvon SD, Lowenthal A, Janz D, et al. Multicenter double-blind, randomized, placebo-controlled trial of levetiracetam as add-on therapy in patients with refractory partial seizures. Epilepsia 2000 Sep; 41(9): 1179–86
Glauser TA, Ayala R, Elterman RD, et al., on behalf of the N159 Study Group. Double blind placebo-controlled trial of adjunctive levetiracetam in pediatric partial seizures. Neurology 2006 Jun; 66(11): 1654–60
Noachtar S, Andermann E, Meyvisch P, et al. Levetiracetam for the treatment of idiopathic generalized epilepsy with myoclonic seizures. Neurology 2008 Feb 19; 70(8): 607–16
Berkovic SF, Knowlton RC, Leroy RF, et al., on behalf of the Levetiracetam N01057 Study Group. Placebo-controlled study of levetiracetam in idiopathic generalized epilepsy. Neurology 2007 Oct; 69(18): 1751–60
French J, di Nicola S, Arrigo C. Fast and sustained efficacy of levetiracetam during titration and the first 3 months of treatment in refractory epilepsy. Epilepsia 2005 Aug; 46(8): 1304–1307
Patsalos PN. Pharmacokinetics profile of levetiracetam: toward ideal characteristics. Pharmacol Ther 2000 Feb; 85(2): 77–85
Perucca E, Gidal BE, Baltes E. Effects of antiepileptic comedication on levetiracetam pharmacokinetics: a pooled analysis of data from randomized adjunctive therapy trials. Epilepsy Res 2003 Feb; 53(1-2): 47–56
Gidal B, Baltes E, Otoul C, et al. Effect of levetiracetam on the pharmacokinetics of adjunctive antiepileptic drugs: a pooled analysis of data from randomized clinical trials. Epilepsy Res 2005 Mar–Apr; 64(1–2): 1–11
Ramael S, De Smedt F, Toublanc N, et al. Single-dose bioavailability of levetiracetam intravenous infusion relative to oral tablets and multiple-dose pharmacokinetics and tolerability of levetiracetam intravenous infusion compared with placebo in healthy subjects. Clin Ther 2006 May; 28(5): 734–44
Pigeolet E, Jacqmin P, Sargentini-Maier ML, et al. Population pharmacokinetics of levetiracetam in Japanese and Western adults. Clin Pharmacokinet 2007; 46(6): 503–12
Strolin Benedetti M, Whomsley R, Nicolas JM, et al. Pharmacokinetics and metabolism of 14C-levetiracetam, a new antiepileptic agent, in healthy volunteers. Eur J Clin Pharmacol 2003 Nov; 59(8–9): 621–30
Pellock JM, Glauser TA, Bebin EM, et al. Pharmacokinetic study of levetiracetam in children. Epilepsia 2001 Dec; 42(12): 1574–9
Fountain NB, Conry JA, Rodriguez-Leyva I, et al. Prospective assessment of levetiracetam pharmacokinetics during dose escalation in 4–12 year old children with partial-onset seizures on concomitant carbamazepine or valproate. Epilepsy Res 2007 Apr; 74(1): 60–9
Glauser TA, Mitchell WG, Weinstock A, et al. Pharmacokinetics of levetiracetam in infants and young children with epilepsy. Epilepsia 2007 Jun; 48(6): 1117–22
Patsalos PN. Clinical pharmacokinetics of levetiracetam. Clin Pharmacokinet 2004; 43(11): 707–24
Glauser TA, Pellock JM, Bebin EM, et al. Efficacy and safety of levetiracetam in children with partial seizures: an open-label trial. Epilepsia 2002 May; 43(5): 518–24
Glauser TA, Gauer LJ, Lu Z, et al. Short and long-term efficacy of levetiracetam adjunctive therapy in children with refractory partial epilepsy. Epilepsia 2005; 46 Suppl. 6: 102
Coupez R, Nicolas JM, Browne TR. Levetiracetam, a new antiepileptic agent: lack of in vitro and in vivo pharmacokinetic interaction with valproic acid. Epilepsia 2003 Feb; 44(2): 171–8
Schwartz GJ, Haycock GB, Edelmann CM, et al. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 1976 Aug; 58(2): 259–63
Mosteller RD. Simplified calculation of body-surface area. N Engl J Med 1987 Oct; 317(17): 1098
Efron B, Gong G. A leisurely look at the bootstrap, the jackknife and the cross-validation. Am Stat 1983; 37: 36–48
US National Center for Health Statistics (NCHS) and US National Center for Chronic Disease Prevention and Health Promotion (NCCDPHP). 2000 CDC growth charts: United States [online]. Hyattsville (MD) and Atlanta (GA): CDC, 2000. Available from URL: http://www.cdc.gov/growthcharts [Accessed 2008 Feb 24]
Rowland M, Tozer N. Clinical pharmacokinetics, concepts and applications. 2nd ed. Philadelphia (PA): Lea & Febiger, 1989: 228
Kearns GL, Abdel-Rahman SM, Alander SW, et al. Developmental pharmacology: drug disposition, action, and therapy in infants and children. N Engl J Med 2003 Sep; 349(12): 1157–67
Coupez R, Straetemans R, Sehgal G, et al. Levetiracetam: relative bioavailability and bioequivalence of a 10% oral solution (750 mg) and 750-mg tablets. J Clin Pharmacol 2003 Dec; 43(12): 1370–6
Tod M, Lokiec F, Bidault R, et al. Pharmacokinetics of oral acyclovir in neonates and in infants: a population analysis. Antimicrob Agents Chemother 2001 Jan; 45(1): 150–7
Anderson BJ, Meakin GH. Scaling for size: some implications for paediatric anaesthesia dosing. Peadiatr Anaesth 2002 Mar; 12(3): 205–19
Mahmood I. Prediction of drug clearance in children from adults: a comparison of several allometric methods. Br J Clin Pharmacol 2006 May; 61(5): 545–57
Johannessen SI, Battino D, Berry DJ, et al. Therapeutic drug monitoring of the newer antiepileptic drugs. Ther Drug Monit 2003 Jun; 25(3): 347–63
May TW, Rambeck B, Jürgens U. Serum concentrations of levetiracetam in epileptic patients: the influence of dose and co-medication. Ther Drug Monit 2003 Dec; 25(6): 690–9
Holford NH. A size standard for pharmacokinetics. Clin Pharmacokinet 1996 May; 30(5): 329–32
Acknowledgements
This study was sponsored by UCB Pharma. Nathalie Toublanc, Maria Laura Sargentini-Maier, Brigitte Lacroix and Armel Stockis are employees of UCB Pharma. Philippe Jacqmin is an employee of Exprimo, an independent company engaged in contract work for UCB Pharma, and has no conflicts of interest that are directly relevant to the content of this study. The assistance of Valéria Molnár in compiling the manuscript is acknowledged.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Toublanc, N., Sargentini-Maier, M.L., Lacroix, B. et al. Retrospective Population Pharmacokinetic Analysis of Levetiracetam in Children and Adolescents with Epilepsy. Clin Pharmacokinet 47, 333–341 (2008). https://doi.org/10.2165/00003088-200847050-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200847050-00004