Abstract
Objective: Ximelagatran, an oral direct thrombin inhibitor, is rapidly bioconverted to melagatran, its active form. The objective of this population analysis was to characterise the pharmacokinetics of melagatran and its effect on activated partial thromboplastin time (APTT), an ex vivo measure of coagulation time, in orthopaedic surgery patients sequentially receiving subcutaneous melagatran and oral ximelagatran as prophylaxis for venous thromboembolism. To support the design of a pivotal dose-finding study, the impact of individualised dosage based on bodyweight and calculated Creatinine clearance was examined.
Design and methods: Pooled data obtained in three small dose-guiding studies were analysed. The patients received twice-daily administration, with either subcutaneous melagatran alone or a sequential regimen of subcutaneous melagatran followed by oral ximelagatran, for 8–11 days starting just before initiation of surgery. Nonlinear mixed-effects modelling was used to evaluate rich data of melagatran pharmacokinetics (3326 observations) and the pharmacodynamic effect on APTT (2319 observations) in samples from 216 patients collected in the three dose-guiding trials. The pharmacokinetic and pharmacodynamic models were validated using sparse data collected in a subgroup of 319 patients enrolled in the pivotal dose-finding trial. The impact of individualised dosage on pharmacokinetic and pharmacodynamic variability was evaluated by simulations of the pharmacokinetic-pharmacodynamic model.
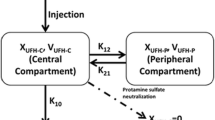
Results: The pharmacokinetics of melagatran were well described by a one-compartment model with first-order absorption after both subcutaneous melagatran and oral ximelagatran. Melagatran clearance was correlated with renal function, assessed as calculated Creatinine clearance. The median population clearance (creatinine clearance 70 mL/min) was 5.3 and 22.9 L/h for the subcutaneous and oral formulations, respectively. The bioavailability of melagatran after oral ximelagatran relative to subcutaneous melagatran was 23%. The volume of distribution was influenced by bodyweight. For a patient with a bodyweight of 75kg, the median population estimates were 15.5 and 159L for the subcutaneous and oral formulations, respectively. The relationship between APTT and melagatran plasma concentration was well described by a power function, with a steeper slope during and early after surgery but no influence by any covariates. Simulations demonstrated that individualised dosage based on Creatinine clearance or bodyweight had no clinically relevant impact on the variability in melagatran pharmacokinetics or on the effect on APTT.
Conclusions: The relatively low impact of individualised dosage on the pharmacokinetic and pharmacodynamic variability of melagatran supported the use of a fixed-dose regimen in the studied population of orthopaedic surgery patients, including those with mild to moderate renal impairment.











Similar content being viewed by others
References
Weite JI, Hirsh J. New anticoagulant drags. Chest 2001; 119 (1 Suppl.): S95–107
Elg M, Gustafsson D, Deinum J. The importance of enzyme kinetics for the effect of thrombin inhibitors in a rat model of arterial thrombosis. Thromb Haemost 1997; 78: 1286–92
Elg M, Gustafsson D, Carlsson S. Antithrombotic effects and bleeding time of thrombin inhibitors and warfarin in the rat. Thromb Res 1999; 94: 187–97
Gustafsson D, Antonsson T, Bylund R, et al. Effects of melagatran, a new low-molecular-weight thrombin inhibitor, on thrombin and fibrinolytic enzymes. Thromb Haemost 1998; 79: 110–8
Mehta JL, Chen L, Nichols WW, et al. Melagatran, an oral active-site inhibitor of thrombin, prevents or delays formation of electrically induced occlusive thrombus in the canine coronary artery. J Cardiovasc Pharmacol 1998; 31: 345–51
Eriksson BI, Carlsson S, Halvarsson M, et al. Antithrombotic effect of two low molecular weight thrombin inhibitors and a low molecular weight heparin in a caval vein thrombosis model in the rat. Thromb Haemost 1997; 78: 1404–7
Mattsson C, Björkman J-A, Ulvinge J-C. Melagatran, hiradin and heparin as adjuncts to tissue-type Plasminogen activator in a canine model of coronary artery thrombolysis. Fibrinolysis Proteolysis 1997; 11: 121–8
Sarich TC, Eriksson UG, Mattsson C, et al. Inhibition of thrombin generation by the oral direct thrombin inhibitor ximelagatran in shed blood from healthy male subjects. Thromb Haemost 2002; 87: 300–5
Eriksson BI, Ögren M, Eriksson UG, et al. Prophylaxis of venous thromboembolism with subcutaneous melagatran in total hip or total knee replacement: results from Phase II studies. Thromb Res 2002; 105: 371–8
Eriksson BI, Arfwidsson A-C, Frison L, et al. A dose-ranging study of the oral direct thrombin inhibitor, ximelagatran, and its subcutaneous form, melagatran, compared with dalteparin in the prophylaxis of thromboembolism after hip or knee replacement: METHRO I. MElagatran for THRombin inhibition in Orthopaedic surgery. Thromb Haemost 2002; 87: 231–7
Eriksson BI, Bergqvist D, Kälebo P, et al. The oral direct thrombin inhibitor, ximelagatran, and its active form, melagatran, in ascending doses compared with dalteparin for prevention of venous thromboembolism after total hip or total knee replacement: The METHRO II Study. Lancet 2002; 360: 1441–7
Eriksson H, Eriksson UG, Frison L, et al. Pharmacokinetics and pharmacodynamics of melagatran, a novel synthetic LMW thrombin inhibitor, in patients with acute DVT. Thromb Haemost 1999; 81: 358–63
Petersen P, SPORTIF II and IV Investigators. A long-term follow-up of ximelagatran as an oral anticoagulant for the prevention of stroke and systemic embolism in patients with atrial fibrillation [abstract]. Blood 2001; 98 Suppl. 1: 706a
Bredberg U, Eriksson UG, Taure K, et al. Effect of melagatran, a novel direct thrombin inhibitor, in healthy volunteers following intravenous, subcutaneous and oral administration [abstract]. Blood 1999; 94 Suppl. 1: 28a
Gustafssson D, Nystrom J, Carlsson S, et al. The direct thrombin inhibitor melagatran and its oral prodrug H 376/95: intestinal absorption properties, biochemical and pharmacodynamic effects. Thromb Res 2001; 101: 171–81
Eriksson UG, Bredberg U, Gislén K, et al. Pharmacokinetics and pharmacodynamics of ximelagatran, a novel oral direct thrombin inhibitor, in young healthy male subjects. Eur J Clin Pharmacol 2003; 59: 35–43
Johansson LC, Frison L, Logren U, et al. The influence of age on the pharmacokinetics and pharmacodynamics of ximelagatran, an oral direct thrombin inhibitor. Clin Pharmacokinet 2003; 42: 381–92
Larsson M, Logren U, Ahnoff M, et al. Determination of melagatran, a novel direct thrombin inhibitor, in human plasma and urine by liquid chromatography: mass spectrometry. J Chromatogr B 2002; 766: 47–55
Beal SL, Sheiner LB. NONMEM users guides. NONMEM Project Group. San Francisco: University of California, 1998
Cockcroft DW, Gault MH. Prediction of Creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41
Cullberg M, Eriksson UG, Larsson M, et al. Population modelling of the effect of inogatran, a thrombin inhibitor, on ex vivo coagulation time (APTT) in healthy subjects and patients with coronary artery disease. Br J Clin Pharmacol 2001; 51: 71–9
Robson R, White H, Aylward P, et al. Bivalirudin pharmacokinetics and pharmacodynamics: effect of renal function, dose and gender. Clin Pharmacol Ther 2002, 9
Acknowledgements
Ulf G. Eriksson, Lars Frisson, Bengt Hamrén and David Gustafsson are employees of AstraZeneca. Jaap W. Mandema and Per Olsson Gisleskog were consultants for AstraZeneca. Mats O. Karlsson received research funding from AstraZeneca. Ulrika Wählby received research funding from and is presently an employee of AstraZeneca. Bengt I. Eriksson was principle investiagtor for the clinical studies and has declared no conflict of interest in connection with this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eriksson, U.G., Mandema, J.W., Karlsson, M.O. et al. Pharmacokinetics of Melagatran and the Effect on Ex Vivo Coagulation Time in Orthopaedic Surgery Patients Receiving Subcutaneous Melagatran and Oral Ximelagatran. Clin Pharmacokinet 42, 687–701 (2003). https://doi.org/10.2165/00003088-200342070-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200342070-00006