Abstract
Background and objective: Oxycodone is a semisynthetic opioid analgesic drug classed as a strong opioid. The controlled-release oxycodone tablet formulation (OCRT) was approved in China in 2004 for management of moderate to severe cancer pain. Few data about the efficacy of OCRT and clinical outcomes in Chinese patients taking this drug are available. The purpose of this study was to evaluate the efficacy and tolerability of this drug for relief of moderate to severe cancer pain in Chinese patients.
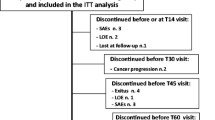
Methods: This was a prospective, open-label, multicentre clinical trial carried out in ten hospitals in Zhejiang Province, China. Patients with cancer pain with a score ≥4 (numerical rating scale) were enrolled. They received oral OCRT at an initial dosage of 5mg every 12 hours for patients scoring 4–6 and 10mg every 12 hours for patients scoring ≥7. Doses were then titrated on an individual basis. Onset of analgesic action, pain score and quality-of-life (QOL) scores —including items measuring family understanding and support, sleep, mental state, appetite, fatigue, and activities of daily life —were evaluated. Adverse effects were also documented.
Results: 216 patients (126 males and 90 females) aged 22–84 years were enrolled. The total mean OCRT dosage was 445.2 ± 361.6mg (range 130–2320mg). The daily dosages of the vast majority of cases (89%) were between 10mg and 30mg. Onset of analgesic action occurred within 1 hour in 198 cases (91.7%) following administration of OCRT. 82.4% of cases were titrated to a steady dosage level within 2 days following administration of the first dose of medication. Pain score decreased significantly (p < 0.01) from 7.1 ± 1.2 at baseline to 2.3 ± 1.2 one week after starting medication and 1.8 ± 0.9 four weeks after starting medication. Scores on all six QOL items increased significantly (p < 0.01) compared with baseline but showed varying rates of improvement. Adverse events included constipation, nausea, vomiting, drowsiness and dysuria. These were noted most frequently in the first week (25.5% of patients) and lessened over time. No severe adverse events were noted.
Conclusion: We conclude that OCRT is well tolerated and effective in controlling moderate to severe cancer pain in Chinese patients.






Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Zhukovsky DS, Abdullah O, Richardson M, et al. Clinical evaluation in advanced cancer. Semin Oncol 2000; 27(1): 14–23
Cherny N. The management of cancer pain. Cancer J Clin 2000; 50: 70–116
Hearn J, Higginson IJ. Cancer pain epidemiology: a systematic review. In: Bruera ED, Portenoy RK, editors. Cancer pain, assessment and management. Cambridge: Cambridge University Press, 2003
Abrahm JL. Promoting symptom control in palliative care. Semin Oncol Nurs 1998; 14(2): 95–109
Mercadante S, Fulfaro F. World Health Organization guidelines for cancer pain: a reappraisal. Ann Oncol 2005; 16 Suppl. 4: ivl32–5
Wootton M. Morphine is not the only analgesic in palliative care: literature review. J Adv Nurs 2004; 45: 527–32
McNicol E, Strassels S, Goudas L, et al. Nonsteroidal anti-inflammatory drugs, alone or combined with opioids, for cancer pain: a systematic review. J Clin Oncol 2004; 22: 1975–92
Stambaugh JE, Reder RF, Stambaugh MD, et al. Double-blind, randomized comparison of the analgesic and pharmacokinetic profiles of controlled- and immediate-release oral oxycodone in cancer pain patients. J Clin Pharmacol 2001; 41(5): 500–6
Kaplan R, Parris WCV, Citron ML, et al. Comparison of controlled-release and immediate-release oxycodone tablets in patients with cancer pain. J Clin Oncol 1998; 16(10): 3230–7
Koizumi W, Toma H, Watanabe K, et al. Efficacy and tolerability of cancer pain management with controlled-release oxycodone tablets in opioid-naive cancer pain patients, starting with 5mg tablets. Jpn J Clin Oncol 2004; 34(10): 608–14
Parris WC, Johnson Jr BW, Croghan MK, et al. The use of controlled-release oxycodone for the treatment of chronic cancer pain: a randomized, double-blind study. J Pain Symptom Manage 1998; 16(4): 205–11
Hagen NA, Babul N. Comparative clinical efficacy and safety of a novel controlled-release oxycodone formulation and controlled-release hydromorphone in the treatment of cancer pain. Cancer 1997; 79(7): 1428–37
Markenson JA, Croft J, Zhang PG, et al. Treatment of persistent pain associated with osteoarthritis with controlled-release oxycodone tablets in a randomized controlled clinical trial. Clin J Pain 2005; 21(6): 524–35
Nicholson B, Ross E, Sasaki J, et al. Randomized trial comparing polymer-coated extended-release morphine sulfate to controlled-release oxycodone HCl in moderate to severe nonmalignant pain. Curr Med Res Opin 2006; 22(8): 1503–14
Zautra AJ, Smith BW. Impact of controlled-release oxycodone on efficacy beliefs and coping efforts among osteoarthritis patients with moderate to severe pain. Clin J Pain 2005; 21(6): 471–7
Hale ME, Fleischmann R, Salzman R, et al. Efficacy and safety of controlled-release versus immediate-release oxycodone: randomized, double-blind evaluation in patients with chronic back pain. Clin J Pain 1999; 15(3): 179–83
Cheville A, Chen A, Oster G, et al. A randomized trial of controlled-release oxycodone during inpatient rehabilitation following unilateral total knee arthroplasty. J Bone Joint Surg Am 2001; 83-A(4): 572–6
Gammaitoni AR, Galer BS, Bulloch S, et al. Randomized, double-blind, placebo-controlled comparison of the analgesic efficacy of oxycodone 10mg/acetaminophen 325mg versus controlled-release oxycodone 20mg in postsurgical pain. J Clin Pharmacol 2003; 43(3): 296–304
de Beer Jde V, Winemaker MJ, Donnelly GA, et al. Efficacy and safety of controlled-release oxycodone and standard therapies for postoperative pain after knee or hip replacement. Can J Surg 2005; 48(4): 277–83
Czarnecki ML, Jandrisevits MD, Theiler SC, et al. Controlled-release oxycodone for the management of pédiatric postoperative pain. J Pain Symptom Manage 2004; 27(4): 379–86
National Comprehensive Cancer Network (NCCN). Adult Cancer Pain, Clinical Practice Guidelines in Oncology, 2005; vol. 2 [online]. Available from URL: http://www.nccn.org/professionals/physician_gls/PDF/pain.pdf [Accessed 2005 Aug 3]
Li Jinxiang, Robert GT, Mellar PD. Palliative medicine.lst ed. Beijing: People’s Health Press, 2005
Cohen MZ, Easley MK, Ellis C, et al. Cancer pain management and the JCAHO’s pain standards: an institutional challenge. J Pain Symptom Manage 2003; 25: 519–27
Kalso E. Oxycodone. J Pain Symptom Manage 2005; 29(5 Suppl.): S47–56
Mucci-LoRusso P, Berman BS, Silberstein PT, et al. Controlled-release oxycodone compared with controlled-release morphine in the treatment of cancer pain: a randomized, double-blind, parallel-group study. Eur J Pain 1998; 2(3): 239–49
Curtis GB, Johnson GH, Clark P, et al. Relative potency of controlled-release oxycodone and controlled-release morphine in a postoperative pain model. Eur J Clin Pharmacol 1999; 55(6): 425–9
Hanks GW, Conno F, Cherny N, et al. Morphine and alternative opioids in cancer pain: the EAPC recommendations. Br J Cancer 2001; 84(5): 587–93
Poyhia R, Vainio A, Kalso E. A review of oxycodone’s clinical pharmacokinetics and pharmacodynamics. J Pain Symptom Manage 1993; 8(2): 63–7
Coluzzi F, Mattia C. Oxycodone: pharmacological profile and clinical data in chronic pain management. Minerva Anestesiol 2005; 71(7–8): 451–60
Gammaitoni AR, Galer BS, Lacouture P, et al. Effectiveness and safety of new oxycodone/acetaminophen formulations with reduced acetaminophen for the treatment of low back pain. Pain Med 2003; 4(1): 21–30
Levy MH. Advancement of opioid analgesia with controlled-release oxycodone. Eur J Pain 2001; 5Suppl. A: 113–6
Lauretti GR, Oliveira GM, Pereira NL. Comparison of sustained-release morphine with sustained-release oxycodone in advanced cancer patients. Br J Cancer 2003; 9(11): 2027–30
Kaiko RF, Benziger DP, Fitzmartin RD, et al. Pharmacokinetic-pharmacodynamic relationships of controlled-release oxycodone. Clin Pharmacol Ther 1996; 59(1): 52–61
Sunshine A, Olson NZ, Colon A, et al. Analgesic efficacy of controlled-release oxycodone in postoperative pain. J Clin Pharmacol 1996; 36(7): 595–603
Gabrail NY, Dvergsten C, Ahdieh H. Establishing the dosage equivalency of oxymorphone extended release and oxycodone controlled release in patients with cancer pain: a randomized controlled study. Curr Med Res Opin 2004; 20(6): 911–8
Hanks GW, Twycross RG, Bliss JM. Controlled-release morphine tablets: a double-blind trial in patients with advanced cancer. Anaesthesia 1987; 42(8): 840–4
Heiskanen TE, Ruismaki PM, Seppala TA, et al. Morphine or oxycodone in cancer pain? Acta Oncol 2000; 39(8): 941–7
Acknowledgements
This study was funded by Sir Run Run Shaw Hospital of Zhejiang University, China. No other sources of funding were used in conducting this study and preparing this manuscript. The authors have no conflicts of interest directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pan, H., Zhang, Z., Zhang, Y. et al. Efficacy and Tolerability of Oxycodone Hydrochloride Controlled-Release Tablets in Moderate to Severe Cancer Pain. Clin. Drug Investig. 27, 259–267 (2007). https://doi.org/10.2165/00044011-200727040-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200727040-00005