Summary
Abstract
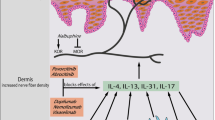
Rupatadine (Rupafin®, Rinialer®, Rupax®, Alergoliber®) is a selective oral histamine H1-receptor antagonist that has also been shown to have platelet-activating factor (PAF) antagonist activity in vitro. It is indicated for use in seasonal allergic rhinitis (SAR), perennial allergic rhinitis (PAR) and chronic idiopathic urticaria (CIU) in patients aged ≥12 years.
Clinical trials show that rupatadine is an effective and generally well tolerated treatment for allergic rhinitis and CIU. It has a rapid onset of action and a prolonged duration of activity. Importantly, it has no significant effect on cognition, psychomotor function or the cardiovascular system. Once-daily rupatadine significantly improves allergic rhinitis symptoms in patients with SAR, PAR or persistent allergic rhinitis (PER) compared with placebo, and provides similar symptom control to that of loratadine, desloratadine, cetirizine or ebastine. In patients with CIU, longer-term use of rupatadine improves CIU symptoms to a greater extent than placebo. It is as well tolerated as other commonly used secondgeneration H1-receptor antagonists. Thus, the introduction of rupatadine extends the range of oral agents available for the treatment of allergic disorders, including allergic rhinitis and CIU.
Pharmacological Properties
Rupatadine shows a similar in vitro affinity for the H1 receptor to loratadine and terfenadine and, like loratadine, is selective for peripheral over central H1 receptors. The drug also demonstrates in vitro affinity for the PAF receptor. The antihistaminic activity and/or anti-inflammatory properties of rupatadine in in vitro studies and in animal models were similar to or greater than those of cetirizine, loratadine, desloratadine, levocabastine or terfenadine. However, unlike these H1 antihistamines, rupatadine also showed specific PAF antagonist activity in vitro and in animal models. In patients with SAR, rupatadine reduced nasal and non-nasal allergen-induced symptoms in an allergen challenge chamber study, and in healthy volunteers, inhibited histamine-induced flare responses. Rupatadine has no clinically relevant effects on the cardiovascular system (in a ‘thorough QT/QTc study’ the risk of QT/QTc prolongation was not increased at rupatadine dosages 10 times the usual dosage in healthy volunteers), cognitive function or psychomotor function.
Rupatadine is rapidly absorbed after oral administration; peak plasma concentrations are achieved within =1 hour and steady state within 3–5 days. Rupatadine is highly protein bound (98–99%) and is well distributed in tissues. It undergoes extensive presystemic hepatic metabolism, predominantly via the cytochrome P450 (CYP) isoenzyme CYP3A4 and is excreted mainly in the bile. Some metabolites are active (e.g. desloratadine and its hydroxylate metabolites) and may contribute to the overall efficacy and long duration of activity of rupatadine. Significant drug interactions between rupatadine and some agents that inhibit CYP3A4 activity occur (e.g. ketoconazole, erythromycin, grapefruit juice) and coadministration of the drug with these agents is not recommended.
Therapeutic Efficacy
Several randomised, placebo- and/or active comparator-controlled trials have shown that rupatadine is effective in improving symptoms in adult or adolescent patients with allergic rhinitis or CIU. In these trials, rupatadine was superior to placebo, showed similar efficacy to loratadine, desloratadine and cetirizine, and was at least as effective as ebastine in reducing allergic symptoms in patients with SAR. Likewise, the drug was also superior to placebo and as effective as loratadine, ebastine or cetirizine in patients with PAR. In patients with PER, rupatadine, but not cetirizine, was superior to placebo at improving allergic symptoms and health-related quality of life (HR-QOL) was improved to a similar extent with rupatadine or cetirizine and to a greater extent than with placebo. Improvements in HR-QOL with rupatadine that were evident in the 4-week double-blind, placebo-controlled phase of another study in patients with PER were maintained for up to 1 year in the open label extension phase. Rupatadine was superior to placebo in improving symptoms of urticaria in patients with CIU; this benefit was evident from the first week of treatment and was maintained throughout a 6-week trial.
Tolerability
Rupatadine was generally well tolerated in clinical trials. A pooled analysis of data from clinical trials indicated that the most common adverse events with rupatadine were somnolence (9.5%), headache (6.8%), fatigue (3.2%), asthenia (1.5%) and dry mouth (1.2%). The majority of adverse events were of mild or moderate severity and there was no significant difference in the overall incidence of adverse events between rupatadine, the active comparators or placebo. The good tolerability of rupatadine was confirmed in a 1-year tolerability study in which the most common adverse event was somnolence (5.8%); headache and dry mouth were each reported by 0.83% of patients.




Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Bousquet J, Van Cauwenberge P, Khaltaev N, et al. Allergic Rhinitis and its Impact on Asthma (ARIA): in collaboration with the World Health Organization. Allergy 2002; 57 (9): 841–55
Kaplan AP. Chronic urticaria: pathogenesis and treatment. J Allergy Clin Immunol 2004 Sep; 114 (3): 465–74
Tedeschi A, Airaghi L, Lorini M, et al. Chronic urticaria: a role for newer immunomodulatory drugs? Am J Clin Dermatol 2003; 4 (5): 297–305
Kozel MM, Sabroe RA. Chronic urticaria: aetiology, management and current and future treatment options. Drugs 2004; 64 (22): 2515–36
Berger WE. The safety and efficacy of desloratadine for the management of allergic disease. Drug Saf 2005; 28 (12): 1101–18
Morgan MM, Khan DA, Nathan RA. Treatment for allergic rhinitis and chronic idiopathic urticaria: focus on oral antihistamines. Ann Pharmacother 2005 Dec; 39(12): 2056–64
Picado C. Rupatadine: pharmacological profile and its use in the treatment of allergic disorders. Expert Opin Pharmacother 2006 Oct; 7 (14): 1989–2001
van Cauwenberge P, Bachert C, Passalacqua G, et al. Consensus statement on the treatment of allergic rhinitis. European Academy of Allergology and Clinical Immunology. Allergy 2000 Feb; 55 (2): 116–34
Dykewicz MS, Fineman S, Skoner P, et al. Diagnosis and management of rhinitis: complete guidelines of the Joint Task Force on Practice Parameters in Allergy, Asthma and Immunology. American Academy of Allergy, Asthma, and Immunology. Ann Allergy Asthma Immunol 1998 Nov; 81 (5 Pt 2): 478–518
Bousquet J, van Cauwenberge P, Aït Khaled N, et al. Pharmacologic and anti-IgE treatment of allergic rhinitis ARIA update (in collaboration with GA2LEN). Allergy 2006 Sep; 61 (9): 1086–96
Zuberbier T, Bindslev-Jensen C, Canonica W, et al. EAACI/GA2LEN/EDF guideline: management of urticaria. Allergy 2006 Mar; 61 (3): 321–31
Broide DH. Molecular and cellular mechanisms of allergic disease. J Allergy Clin Immunol 2001; 108 (2): S65–71
Greiner AN, Meltzer EO. Pharmacologic rationale for treating allergic and nonallergic rhinitis. J Allergy Clin Immunol 2006 Nov; 118 (5): 985–96
Hennino A, Bérard F, Guillot I, et al. Pathophysiology of urticaria. Clin Rev Allergy Immunol 2006 Feb; 30 (1): 3–11
Horak F. Clinical advantages of dual activity in allergic rhinitis. Allergy 2000; 55 Suppl. 64: 34–9
Merlos M, Giral M, Balsa D, et al. Rupatadine, a new potent, orally active dual antagonist of histamine and platelet-activating factor (PAF). J Pharmacol Exp Ther 1997 Jan; 280 (1): 114–21
García-Rafanell J. Rupatadine fumarate: antiallergic, histamine and PAF antagonist. Drugs Fut 1996 Oct; 21 (10): 1033–6
Uriach J, Cia SA. Rupafin 10mg comprimidos (rupatadina): resumen de las características del producto [online]. Available from URL: http://www.msc.es/profesional/farmacia/informaMedicamentos/doc/F_TecnicaNum64053.doc [Accessed 2004 Feb 3]
Merlos M, Balsa D, Giral M, et al. Inhibition of rat peritoneal mast cell exocytosis by rupatadine fumarate: a study with different secretagogues [abstract no. PA-95]. Methods Find Exp Clin Pharmacol 1997; 19 Suppl. A: 148
Giral M, Balsa D, Ferrando R, et al. CNS activity profile of rupatadine fumarate, a new dual receptor antagonist of platelet-activating factor and histamine [abstract no. P321]. Allergy 1998; 53 Suppl. 43: 131–2
Izquierdo I, Merlos M, Garcia-Rafanell J. Rupatadine, a new selective histamine H1 receptor and platelet-activating factor (PAF) antagonist: a review of pharmacological profile and clinical management of allergic rhinitis. Drugs Today (Barc) 2003 Jun; 39(6): 451–68
Queralt M, Brazis P, Merlos M, et al. Inhibitory effects of rupatadine on mast cell histamine release and skin wheal development induced by Ascaris suum in hypersensitive dogs. Drug Dev Res 1998; 44 (2–3): 49–55
Queralt M, Brazis P, Merlos M, et al. In vitro inhibitory effect of rupatadine on histamine and TNF-α release from dispersed canine skin mast cells and the human mast cell line HMC-1. Inflamm Res 2000 Jul; 49(7): 355–60
Queralt M, Merlos M, Giral M, et al. Dual effect of a new compound, rupatadine, on edema induced by platelet-activating factor and histamine in dogs: comparison with antihistamines and PAF antagonists. Drug Dev Res 1996; 39(1): 12–8
Ferrando R, Giral M, Balsa D, et al. Protective effect of rupatadine fumarate in experimental conjunctivitis in guinea pigs [abstract no. P-21]. Methods Find Exp Clin Pharmacol 1996; 18 Suppl. B: 140
Merlos M, Giral M, Balsa D, et al. Rupatadine inhibits the eosinophil recruitment in BAL fluid of ovalbumin-sensitized guinea pigs [abstract no. 904]. J Allergy Clin Immunol 1998 Jan; 101 (Pt 2): S218
Stuebner P, Horak F, Zieglmayer R, et al. Effects of rupatadine vs placebo on allergen-induced symptoms in patients exposed to aeroallergens in the Vienna Challenge Chamber. Ann Allergy Asthma Immunol 2006 Jan; 96 (1): 37–44
Izquierdo I, Nieto C, Ramis J, et al. Pharmacokinetics and dose linearity of rupatadine fumarate in healthy volunteers [abstract no. PB-62]. Methods Find Exp Clin Pharmacol 1997; 19 Suppl. A: 189–203
Barrón S, Ramis I, Merlos M. Effect of rupatadine on lymphocyte cytokine production [abstract no. 1175]. Allergy Clin Immunol Int 2005; Suppl. 1: 427
Taglialatela M, Annunziato I. Evaluation of the cardiac safety of second-generation antihistamines. Allergy 2000; 55: 22–30
Caballero R, Valenzuela C, Longobardo M, et al. Effects of rupatadine, a new dual antagonist of histamine and plateletactivating factor receptors, on human cardiac Kv1.5 channels. BrJ Pharmacol 1999 Nov; 128 (5): 1071–81
Izquierdo I, Pérez I, Villa M. Lack of electrocardiographic effects of Rupatadine, new nonsedating selective histamine HI-receptor and PAF antagonist [abstract no. 637]. Allergy 2001; 56 Suppl. 68: 212
Ramis I, Giral M, Nieto C, et al. Lack of cardiotoxic effects of rupatadine in isolated Purkinje fibres and its relationship with rupatadine cardiac levels [abstract no. 947]. Allergy 2000; 55 Suppl. 63: 264
Giral M, Merlos M, Balsa D, et al. Effects of rupatadine on cardiovascular profile in rats and guinea pigs: comparison with other nonsedating antihistamines [abstract no. 110]. Allergy 1997; 52 Suppl. 37: 44
E14: The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. ICH Harmonised Tripartite Guideline. Current Step 4 version. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, 2005 May 12 [online]. Available from URL: http://www.ich.org [Accessed 2006 Nov 16]
Donado E, Pérez I, García O, et al. Cardiac safety of rupatadine according to the new ICH guideline: a “thorough QT/QTc study” [abstract no. 760 plus poster]. The XXV Congress of the European Academy of Allergology and Clinical Immunology; 2006 Jun 10–14; Vienna
Barbanoj MJ, García-Gea C, Morte A, et al. Central and peripheral evaluation of rupatadine, a new antihistamine/plateletactivating factor antagonist, at different doses in healthy volunteers. Neuropsychobiology 2004; 50 (4): 311–21
Vuurman E, Theunissen E, van Oers A. Lack of effects between rupatadine 10mg and placebo on actual driving performance of healthy volunteers. Human Psychopharmacol Clin Exp. In press
Barbanoj MJ, García-Gea C, Antonijoan R, et al. Evaluation of the cognitive, psychomotor and pharmacokinetic profiles of rupatadine, hydroxyzine and cetirizine, in combination with alcohol, in healthy volunteers. Hum Psychopharmacol Clin Exp 2006 Jan; 21 (1): 13–26
Solans A, Merlos M, Antonijoan R, et al. Pharmacokinetic and safety profile of rupatadine when coadministered with azithromycin: a randomized, crossover, multiple-dose open study [abstract no. 410]. Allergy Clin Immunol Int 2005; Suppl. 1: 159
Izquierdo I, Paredes I, Lurigados C, et al. A dose ranging study of rupatadine fumarate in patients with seasonal allergic rhinitis [abstract no. 984]. Allergy 2000; 55 Suppl. 63: 275
Pérez I, De la Cruz G, Villa M, et al. Rupatadine in allergic rhinitis: pooled analysis of efficacy data [abstract no. 784]. Allergy 2002; 57 Suppl. 73: 245
Saint-Martin F, Dumur JP, Pérez I, et al. A randomized, double-blind, parallel-group study, comparing the efficacy and safety of rupatadine (20 and 10 mg), a new PAF and H1 receptorspecific histamine antagonist, to loratadine 10 mg in the treatment of seasonal allergic rhinitis. J Investig Allergol Clin Immunol 2004; 14 (1): 34–40
Guadaño EM, Serra-Batlles J, Meseguer J, et al. Rupatadine 10 mg and ebastine 10 mg in seasonal allergic rhinitis: a comparison study. Allergy 2004 Jul; 59(7): 766–71
Martinez-Cócera C, De Molina M, Marti-Guadaño E, et al. Rupatadine 10 mg and cetirizine 10 mg in seasonal allergic rhinitis: a randomised, double-blind parallel study. J Investig Allergol Clin Immunol 2005; 15 (1): 22–9
Maspero J, Fantin S, Bisbal C, et al. A 12-week placebocontrolled study of rupatadine 10mg once daily comparative with cetirizine 10mg once daily, in the treatment of persistent allergic rhinitis [abstract no. 758 plus poster]. The XXV Congress of the European Academy of Allergology and Clinical Immunology; 2006 Jun 10–14; Vienna
Roger A, Arnáiz E, Valero A, et al. Rupatadine 10mg improves quality of life in long-term treatment of persistent allergic rhinitis [abstract no. 761 plus poster]. The XXV Congress of the European Academy of Allergology and Clinical Immunology; 2006 Jun 10–14; Vienna
Dubertret L, Zalupca L, Cristodoulo T, et al. Once-daily rupatadine improves the symptoms of chronic idiopathic urticaria: a randomised, double-blind, placebo-controlled study. Eur J Dermatol. In press
Giménez-Arnau A, Pujol RM, Ianosi S, et al. Rupatadine in the treatment of chronic idiopathic urticaria: a double-blind, randomized, placebo-controlled multicenter study. Allergy. In press
Juniper EF, Thompson AK, Ferrie PJ, et al. Development and validation of the standardized Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ(S)) [abstract no. 273]. J Allergy Clin Immunol 1999; 103 (1 Pt 2): S71
Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol 1994 May; 19(3): 210–6
El: The extent of population exposure to assess clinical safety for drugs intended for long-term treatment of non-life-threatening conditions. ICH Harmonised Tripartate Guideline. Current Step 4 version. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use [online]. Available from URL: http://www.ich.org/LOB/media/MEDIA435.pdf [Accessed 2006 Nov 23]
European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP). Guideline on the clinical development of medicinal products for the treatment of allergic rhinoconjunctivitis [online]. Available from URL: http://www.emea.eu.int/pdfs/human/ewp/245502en.pdf [Accessed 2006 Nov 23]
Valero A, Rivas P, Castillo JA, et al. One year safety data of rupatadine 10mg in patients with allergic rhinitis [abstract no. 759 plus poster]. The XXV Congress of the European Academy of Allergology and Clinical Immunology; 2006 Jun 10–14; Vienna
Salmun LM. Antihistamines in late-phase clinical development for allergic disease. Expert Opin Investig Drugs 2002 Feb; 11 (2): 259–73
Baiardini I, Braido F, Cauglia S, et al. Sleep disturbances in allergic diseases. Allergy 2006 Nov; 61 (11): 1259–67
Monroe E. Review of H1 antihistamines in the treatment of chronic idiopathic urticaria. Cutis 2005 Aug; 76 (2): 118–26
Salib RJ, Howarth PH. Safety and tolerability profiles of intranasal antihistamines and intranasal corticosteroids in the treatment of allergic rhinitis. Drug Saf 2003; 26 (12): 863–93
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: C. Colás, Department of Allergy, Hospital Clinico Universitario “Lozano Blesa”, Zarazoga, Spain; S.E. Fantin, Hospital President Peron, Buenos Aires, Argentina; F. Horak, Allergy Centre Vienna West, Vienna, Austria; J. Maspero, Fundación CIDEA, Buenos Aires, Argentina; C. Picado, Department of Pneumology and Respiratory Allergy, University of Barcelona, Hospital Clinic i Provincial de Barcelona, Barcelona, Spain; A. Valero, Department of Pneumology and Respiratory Allergy, Hospital Clinic i Provincial de Barcelona, Barcelona, Spain; E. Vuurman, Experimental Psychopharmacology Unit, Brain & Behavior Institute, Universiteit Maastricht, Maastricht, The Netherlands.
Data Selection
Sources: Medical literature published in any language since 1980 on ‘rupatadine’, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database of Wolters Kluwer Health | Adis). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE, EMBASE and AdisBase search term was ‘rupatadine’. Searches were last updated 1 February 2007.
Selection: Studies in patients with allergic rhinitis or chronic idiopathic urticaria who received rupatadine. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Rupatadine, histamine H1-receptor antagonist, allergic rhinitis, chronic idiopathic urticaria, pharmacodynamics, pharmacokinetics, therapeutic use.
Rights and permissions
About this article
Cite this article
Keam, S.J., Plosker, G.L. Rupatadine. Drugs 67, 457–474 (2007). https://doi.org/10.2165/00003495-200767030-00008
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200767030-00008