Abstract
Background
The National Centre for Pharmacoeconomics, in collaboration with the Health Services Executive, considers the cost effectiveness of all new medicines introduced into Ireland. Health Technology Assessments (HTAs) are conducted in accordance with the existing agreed Irish HTA guidelines. These guidelines do not specify a formal analysis of value of information (VOI).
Objective
The aim of this study was to demonstrate the benefits of using VOI analysis in decreasing decision uncertainty and to examine the viability of applying these techniques as part of the formal HTA process for reimbursement purposes within the Irish healthcare system.
Method
The evaluation was conducted from the Irish health payer perspective. A lifetime model evaluated the cost effectiveness of rivaroxaban, dabigatran etexilate and enoxaparin sodium for the prophylaxis of venous thromboembolism after total hip replacement.
The expected value of perfect information (EVPI) was determined directly from the probabilistic analysis (PSA). Population-level EVPI (PEVPI) was determined by scaling up the EVPI according to the decision incidence. The expected value of perfect parameter information (EVPPI) was calculated for the three model parameter subsets: probabilities, preference weights and direct medical costs.
Results
In the base-case analysis, rivaroxaban dominated both dabigatran etexilate and enoxaparin sodium. PSA indicated that rivaroxaban had the highest probability of being the most cost-effective strategy over a threshold range of €0-€100 000 per QALY. At a threshold of €45 000 per QALY, the probability that rivaroxaban was the most cost-effective strategy was 67%.
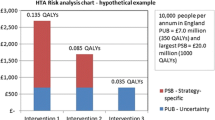
At a threshold of €45 000 per QALY, assuming a 10-year decision time horizon, the PEVPI was €11.96 million and the direct medical costs subset had the highest EVPPI value (€9.00 million at a population level). In order to decrease uncertainty, a more detailed costing study was undertaken.
In the subsequent analysis, rivaroxaban continued to dominate both comparators. In the PSA, rivaroxaban continued to have the highest probability of being optimal over the threshold range €0-€100 000 per QALY. At €45 000 per QALY, the probability that rivaroxaban was the most cost-effective strategy increased to 80%.
At €45 000 per QALY, the 10-year PEVPI decreased to €3.58 million and the population value associated with the direct medical costs fell to €1.72 million.
Conclusion
This increase in probability of cost effectiveness, coupled with a substantially reduced potential opportunity loss could influence a decision maker’s confidence in making a reimbursement decision. On discussions with the decision maker we now intend to incorporate the use of VOI into our HTA process.









Similar content being viewed by others
References
Xie F, Blackhouse G, Assasi N, et al. Results of a model analysis to estimate cost utility and value of information for intravenous immunoglobulin in Canadian adults with chronic immune thrombocytopenic purpura. Clin Ther 2009; 31 (5): 1082–91.
Hoomans T, Fenwick E, Palmer S, et al. Value of information and value of implementation: application of an analytic framework to inform resource allocation decisions in metastatic hormone-refractory prostate cancer. Value Health 2009; 12 (2): 315–24.
Claxton K. Exploring uncertainty in cost-effectiveness analysis. Pharmacoeconomics 2008; 26 (9): 781–98.
Barton G, Briggs A, Fenwick E. Optimal cost-effectiveness decisions: the role of the cost-effectiveness acceptability curve (CEAC), the cost-effectiveness acceptability frontier (CEAF), and the expected value of perfection information (EVPI). Value Heath 2008; 11 (5): 886–97.
Groot-Koerkamp B, Hunink M, Stijnen T, et al. Limitations of acceptability curves for presenting uncertainty in cost-effectiveness analysis. Med Decis Making 2007; 27: 101–11.
Barton P. What happens to value of information measures as the number of decision options increases? Health Econ 2011; 20 (7): 853–63.
Fenwick E, Palmer S, Claxton K, et al. An iterative Bayesian approach to health technology assessment: application to a policy of preoperative optimization for patients undergoing major elective surgery. Med Decis Making 2006; 26: 480–96.
Groot-Koerkamp B, Hunink M, Stijnen T, et al. Identifying key parameters in cost-effectiveness analysis using value of information: a comparison of methods. Health Econ 2006; 15: 383–92.
Rojnik K, Naveršnik K. Gaussian process metamodeling in Bayesian value of information analysis: a case of the complex health economic model for breast cancer screening. Value Health 2008; 11 (2): 240–50.
Ades A, Lu G, Claxton K. Expected value of sample information in medical decision modelling. Med Dec Making 2004; 24: 207–72.
Claxton K, Neumann P, Araki S, et al. Bayesian value of information analysis: an application to a policy model of Alzheimer’s disease. Int J Technol Assess Health Care 2001; 17: 38–55.
Brennan A, Kharroubi S, O’Hagan A, et al. Calculating partial expected value of perfect information via Monte Carlo sampling algorithms. Med Decis Making 2007; 27: 448–70.
Eckermann S, Karnon J, Willan AR. The value of value of information: best informing research design and prioritization using current methods. PharmacoEconomics 2010; 28 (9): 699–709.
Health Information and Quality Authority. Guidelines for the economic evaluation of health technologies in Ireland. Cork and Dublin, Ireland, 2010 [online]. Available from URL: http://www.hiqa.ie/healthcare/health-technology-assessment/guidelines [Accessed 2010 Nov 12].
Sculpher M, Claxton K. Establishing the cost-effectiveness of new pharmaceuticals under conditions of uncertainty: when is there sufficient evidence? Value Health 2005; 8 (4): 433–66.
National Collaborating Centre for Acute Care. Venous thromboembolism: reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in inpatients undergoing surgery. Commissioned by the National Institute for Health and Clinical Excellence (NICE); London, April 2007 [online]. Available from URL: http://www.nice.org.uk/CG046fullguideline [Accessed 2008 Aug 1].
Geerts W, Bergqvist D, Pineo G, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133 (6 Suppl.): 381s–453s.
Freedman K, Brookenthal K, Fitzgerald R, et al. A metaanalysis of thromboembolic prophylaxis following elective total hip arthroplasty. J Bone Joint Surg Am 2000; 82-A (7): 929–38.
Cardiovascular Disease Educational and Research Trust, Cyprus Cardiovascular Disease Educational and Research Trust, European Venous Forum, International Surgical Thrombosis Forum, International Union of Angiology, Union Internationale de Phlebologie. Prevention and treatment of venous thromboembolism. International Consensus Statement (guidelines according to scientific evidence). Int Angiol 2006; 25 (2): 101–61.
Carter C. The natural history and epidemiology of venous thrombosis. Prog Cardiovasc Dis 1994; 36: 423–38.
Scarvelis D, Wells P. Diagnosis and treatment of deep-vein thrombosis. CMAJ 2006; 175 (9): 1–6.
Hansson P, Sorbo J, Eriksson H. Recurrent venous thromboembolism after deep vein thrombosis. Arch Intern Med 2000; 160: 769–74.
Prandoni P, Villata S. The clinical course of deep vein thrombosis, prospective long term follow up of 528 symptomatic patients. Haematologica 1997; 82: 423–8.
Prandoni P, Lensing A, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002; 100 (10): 3484–8.
Prandoni P, Lensing A, Prins M, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med 2002; 137 (12): 955–60.
Ziegler S, Schillinger T, Minar E. Post-thrombotic syndrome after primary event of deep vein thrombosis 10 to 20 years ago. Thromb Res 2001; 101: 23–33.
Beyth R, Cohen A, Landefeld C. Long term outcomes of deep vein thrombosis. Arch Intern Med 1995; 155 (155): 1031–7.
Ginsberg J, Gent M, Turkstra F, et al. Post-thrombotic syndrome after hip or knee arthroplasty: a cross sectional study. Arch Intern Med 2000; 160: 669–72.
Prandoni P, Bernardi E, Marchiori A, et al. The long term clinical course of acute deep vein thrombosis of the arm: prospective cohort study. BMJ 2004; 2004 (329): 484–5.
Barry M, Tilson L. Recent developments in pricing and reimbursement of medicines in Ireland. Expert Rev Pharmacoecon Outcomes Res 2007; 7 (6): 605–11.
Botteman M, Caprini J, Stephens J, et al. Results of an economic model to access the cost-effectiveness of enoxaparin, a low molecualr weight heparin, versus warfarin for the prophylaxis of deep vein thrombosis and associated long term complications in total hip replacement surgery in the United States. Clin Ther 2002; 24 (11): 1960–86.
Wolowacz S, Roskell N, Maciver F, et al. Economic evaluation of dabigatran etexilate for the prevention of venous thromboembolism after total knee and hip replacement surgery. Clin Ther 2009; 31 (1): 194–212.
McCullagh L, Tilson L, Walsh C, et al. A cost-effectiveness model comparing rivaroxaban and dabigatran etexilate with enoxaparin sodium as thromboprophylaxis after total hip and total knee replacement in the Irish healthcare setting. Pharmacoeconomics 2009; 27 (10): 829–46.
Caprini J, Botteman M, Stephens J, et al. Economic burden of long-term complications of deep vein thrombosis after total hip replacement surgery in the United States. Value Health 2003; 6 (1): 59–74.
Eriksson B, Dahl O, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for the prevention of venous thromboembolism after total hip replacement: a randomised, double blind, non-inferiority trial. The RE-NOVATE Study group. Lancet 2007; 370: 949–56.
Kakkar A, Brenner B, Dahl O, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. The RECORD 2 Investigators. Lancet 2008; 372 (9632): 31–9.
Bucher H, Guyatt G, Griffith L, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997; 50: 683–91.
Heit J, Rooke T, Silverstein M, et al. Trends in the incidence of venous stasis syndrome and venous ulcer: a 25 year population-based study. J Vasc Surg 2001; 33: 1022–7.
Wille-Jorgensen P, Jorgensen L, Crawford M. Asymptomatic postoperative deep vein thrombosis and the development of post-thrombotic syndrome. Thromb Haemost 2005; 93: 236–41.
Central Statistics Office. Irish Life Tables: life expectancy by age, 2005–2007. 29th January 2009 [online]. Available from URL: http://www.cso.ie [Accessed 2009 Feb 17].
Oster G, Tuden RL, Colditz GA. A cost-effectiveness analysis of prophylaxis against deep-vein thrombosis in major orthopaedic surgery. JAMA 1987; 257: 203–8.
Menzin J, Colditz G, Regan M, et al. Cost effectiveness of enoxaparin vs low dose warfarin in the prevention of deep-vein thrombosis after total hip replacement surgery. Arch Intern Med 1995; 155: 757–64.
Nerurkar J, Wade W, Martin C. Cost/death averted with venous thromboembolism prophylaxis in patients undergoing total knee replacement or knee arthroplasty. Pharmacotherapy 2002; 22 (8): 990–1000.
Rubinstein I, Murray D, Hoffstein V. Fatal pulmonary emboli in hospitalized patients: an autopsy study. Ann Intern Med 1988; 148 (6): 1425–6.
Douketis J, Kearon C, Bates S. Risk of fatal pulmonary embolism in patients with treated venous thromboembolism. JAMA 1998; 279 (6): 458–62.
Stein P, Andjerald W, Henry M. Prevalence of acute pulmonary embolism among patients in a general hospital and at autopsy. Chest 1995; 108: 978–81.
Lagerstedt C, Olsson C, Fagher BO. Need for long term anticoagulant therapy in symptomatic calf vein thrombosis. Lancet 1985; 2: 515–8.
Moser K, LeMoine JR. Is embolic risk conditioned by location of deep venous thrombosis? Ann Intern Med 1981; 94 (4 Pt 1): 439–44.
White R, Romano P, Zhou H. Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med 1998; 158: 1525–31.
Fryback D, Lawrence W. Dollars may not buy as many QALYS as we think: a problem with defining quality of life adjustments. Med Decis Making 1997; 17: 276–84.
Lenert L, Soetikno R. Automated computer interviews to elicit utilities: potential application in the treatment of deep vein thrombosis. J Am Med Inform Assoc 1997; 4 (1): 49–56.
Monreal M, Martorell A, Callejas J, et al. Venographic assesment of deep vein thrombosis and risk of developing post-thrombotic syndrome: a prospective study. J Intern Med 1993; 233: 233–8.
Cykert S, Phifer N, Hansen C. Tamoxifen for breast cancer prevention: a framework for clinical decisions. Obstet Gynecol 2004; 104 (3): 433–42.
Casemix. Ready reckoner of acute hospital inpatient activity and costs (summarised by DRG) relating to 2005 costs & activity (part 3). Dublin: Health Services Executive (HSE), 2007.
Youman P, Wilson K, Harraf F, et al. The economic burden of stroke in the United Kingdom. Pharmacoeconomics 2003; 21: 43–50.
Central Statistics Office Ireland. Consumer Price Index for Health [online]. Available from URL: http://www.cso.ie/statistics/consumerpriceindex.htm [Accessed 2010 Oct 01].
Currency Calculator. October 2008 — 23-day average [online]. Available from URL: http://www.x-rates.com [Accessed 2009 Feb 1].
Health Services Executive (HSE). Primary Care Reimbursement Service (PCRS) reimbursable items. 2010 [online]. Available from URL: http://www.sspcrs.ie/druglist/search.jsp [Accessed 2010 Oct 01].
Monthly Index of Medical Specialities (MIMS). Ireland. Dublin: Medical Publications (Ireland). Available from URL: www.mims.ie [Accessed 2008 Oct 1].
Casemix. Ready reckoner of acute hospital inpatient activity and costs (summarised by DRG) relating to 2008 costs and activity. Dublin: Health Service Executive (HSE), 2010.
Irish Heart Foundation. Cost of stroke in Ireland: estimating the annual economic cost of stroke and transient ischaemic attack (TIA) in Ireland. Report prepared for the Irish Heart Foundation by the Economic and Social Research Institute (ESRI) and the Royal College of Surgeons of Ireland (RCSI). September 2010 [online]. Available from URL: http://www.esri.ie/UserFiles/publications/bkmnext170.pdf [Accessed 2010 Oct 18].
Molony S, Molony D, O’Leary A. Clinical audit of the management of patients in an anticoagulant primary care clinic in Ireland. Dublin, Ireland: Senior Cycle Research, School of Pharmacy, Royal College of Surgeons of Ireland (RCSI), 2009.
Department of Public Expenditure and Reform. Project discount and inflation rates. [online]. Available from URL: http://per.gov.ie/project-discount-inflation-rates/ [Accessed 2012 May 16].
Gelman A, Carlin J, Stern H, et al. Bayesian data analysis. London: Chapman & Hall, 1995.
Briggs A, Ades A, Price M. Probabilistic sensitivity analysis for decision trees with multiple branches: use of the Dirichlet distribution in a Bayesian framework. Med Decis Making 2003; 23: 341–50.
O’Hagan A, Luce B. A primer on Bayesian statistics in health economics and outcomes research. Bethesda (MD): MEDTAP International, Inc., 2003.
Garthwaite PH, Kadane JB, O’Hagan A. Statistical methods for eliciting probability distributions. J Am Stat Assoc 2005; 100 (470): 680–701.
Johannesson M, Weinstein M. On the decision rules of cost-effectiveness analysis. J Health Econ 1993; 12: 459–67.
Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001; 10: 779–87.
Bojke L, Claxton K, Sculpher M, et al. Identifying research priorities: the value of information associated with repeat screening for age-related macular degeneration. Med Decis Making 2008; 28 (33): 33–43.
Claxton K. The irrelevance of interference: a decisionmaking approach to the stochastic evaluation of health care technologies. J Health Econ 1999; 18: 341–64.
Health Research and Information Division (HRID), The Economic and Social Research Institute (ESRI). Activity in acute public hospitals in Ireland, 2008 annual report [online]. Available from URL: http://www.esri.ie/health_information/latest_hipe_nprs_reports/2008/2008_HIPE_Annual_Report_-_Final.pdf [Accessed 2010 Sep 1].
Cohen A, Tapson V, Bergmann J. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE Study): a multinational cross-sectional study. Lancet 2008; 371: 387–94.
Claxton K, Sculpher M. Using value of information analysis to prioritise health research: some lessons from recent UK experience. Pharmacoeconomics 2006; 24 (11): 1055–68.
Bojke L, Claxton K, Bravo-Vergel Y, et al. Eliciting distributions to populate decision analytic models. Value Health 2010; 13 (5): 557–64.
Garthwaite P, Chilcott J, Jenkinson D, et al. Use of expert knowledge in evaluating costs and benefits of alternative service provisions: a case study. Int J Technol Assess Health Care 2008; 24 (3): 350–7.
Wailoo A, Sutton A, Cooper N, et al. Cost-effectiveness and value of information analyses of neuraminidase inhibitors for the treatment of influenza. Value Health 2008; 11 (2): 160–71.
Yokota F, Thompson K. Value of information literature analysis: a review of applications in health risk management. Med Dec Making 2004; 24: 287–98.
Philips Z, Claxton K, Palmer S. The half-life of truth: what are appropriate time horizons for research decisions? Med Decis Making 2008; 28: 287–99.
Poynard T, Munteanu M, Ratziu V, et al. Truth survival in clinical research: an evidence-based requiem? Ann Intern Med 2002; 136: 888–95.
LaValley MP, Felson D. Truth survival [letter]. Ann Intern Med 2002; 137 (11): 932.
Tappenden P, Chilcott J, Eggington J, et al. Methods for expected value of information analysis in complex health economic models: developments on the health economics of interferon-beta and glatiramer acetate for multiple sclerosis. Health Technol Assess 2004; 8 (27): 1–78.
Oakley JE, Brennan A, Tappenden P, et al. Simulation sample sizes for Monte Carlo partial EVPI calculations. J Health Econ 2010; 29 (3): 468–77.
Oostenbrink J, Al MJ, Oppe M, et al. Expected value of perfect information: an empirical example of reducing decision uncertainty by conducting additional research. Value Health 2008; 11 (7): 1070–80.
Acknowledgements
No sources of funding were used to conduct this study or prepare this manuscript. The authors have no conflicts of interest that are directly relevant to the content of this article.
Ms McCullagh was involved in the conception, planning and management of the study. Ms McCullagh developed the initial cost-effectiveness model structure, interpreted the results and prepared the first draft of the manuscript. Prof. Walsh contributed to the conception and planning, the statistical design and analysis, and the interpretation of results. Prof. Walsh also reviewed the manuscript for important intellectual content. Prof. Barry was involved in the conception and planning of the work; he critically revised the manuscript and approved the final submitted version. Prof. Barry also provided clinical input throughout. Ms McCullagh acts as a guarantor for the overall manuscript content.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McCullagh, L., Walsh, C. & Barry, M. Value-of-Information Analysis to Reduce Decision Uncertainty Associated with the Choice of Thromboprophylaxis after Total Hip Replacement in the Irish Healthcare Setting. PharmacoEconomics 30, 941–959 (2012). https://doi.org/10.2165/11591510-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11591510-000000000-00000