Abstract
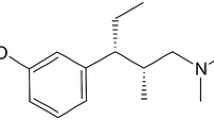
Background: Tapentadol is a novel, centrally acting analgesic with μ-opioid receptor agonist and norepinephrine reuptake inhibitor activity.
Objective: To evaluate the efficacy and safety of tapentadol extended release (ER) compared with oxycodone controlled release (CR) for management of moderate to severe chronic osteoarthritis-related knee pain.
Methods: This was a randomized, double-blind, active- and placebo-controlled, parallel-arm, multicentre, phase III study during which patients received tapentadol ER, oxycodone CR or placebo for a 3-week titration period followed by a 12-week maintenance period. The study was carried out at sites in Australia, Canada, New Zealand and the US. A total of 1030 patients with chronic osteoarthritis-related knee pain were randomized to receive tapentadol ER 100–250 mg twice daily, oxycodone HCl CR 20–50 mg twice daily or placebo. Primary endpoints (as determined prior to initiation of the study) were the changes from baseline in average daily pain intensity (rated by patients on an 11-point numerical rating scale) over the last week of maintenance and over the entire 12-week maintenance period; last observation carried forward was used to impute missing values after early treatment discontinuation.
Results: Efficacy and safety were evaluated for 1023 patients. Tapentadol ER significantly reduced average pain intensity from baseline to week 12 of the maintenance period versus placebo (least squares mean [LSM] difference [95% CI], −0.7 [−1.04, −0.33]), and throughout the maintenance period (−0.7 [−1.00, −0.33]). Oxycodone CR significantly reduced average pain intensity from baseline throughout the maintenance period versus placebo (LSM difference [95% CI], −0.3 [−0.67, −0.00]) but not at week 12 (−0.3 [−0.68, 0.02]). A significantly higher percentage of patients achieved ≥50% improvement in pain intensity in the tapentadol ER group (32.0% [110/344]) compared with the placebo group (24.3% [82/337]; p = 0.027), indicating a clinically significant improvement in pain intensity, while a significantly lower percentage of patients achieved ≥50% improvement in pain intensity in the oxycodone CR group (17.3% [59/342]; p = 0.023 vs placebo). In the placebo, tapentadol ER and oxycodone CR groups, respectively, 61.1% (206/337), 75.9% (261/344) and 87.4% (299/342) of patients reported at least one treatment-emergent adverse event (TEAE); incidences of gastrointestinal-related TEAEs were 26.1% (88/337), 43.0% (148/344) and 67.3% (230/342).
Conclusion: Treatment with tapentadol ER 100–250 mg twice daily or oxycodone HCl CR 20–50 mg twice daily was effective for the management of moderate to severe chronic osteoarthritis-related knee pain, with substantially lower incidences of gastrointestinal-related TEAEs associated with treatment with tapentadol ER than with oxycodone CR. [Trial registration number: NCT00421928 (ClinicalTrials.gov Identifier)]










Similar content being viewed by others
References
Corti MC, Rigon C. Epidemiology of osteoarthritis: prevalence, risk factors and functional impact. Aging Clin Exp Res 2003 Oct; 15(5): 359–63
Breedveld FC. Osteoarthritis: the impact of a serious disease. Rheumatology (Oxford) 2004 Feb; 43Suppl. 1: i4–8
Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 2008 Feb; 16(2): 137–62
Hunter DJ, Felson DT. Osteoarthritis. BMJ 2006 Mar 18; 332(7542): 639–42
Gibofsky A, Barkin RL. Chronic pain of osteoarthritis: considerations for selecting an extended-release opioid analgesic. Am J Ther 2008 May; 15(3): 241–55
Brandt KD. The role of analgesics in the management of osteoarthritis pain. Am J Ther 2000 Mar; 7(2): 75–90
Katz WA. Use of nonopioid analgesics and adjunctive agents in the management of pain in rheumatic diseases. Curr Opin Rheumatol 2002 Jan; 14(1): 63–71
Mamdani M, Rochon PA, Juurlink DN, et al. Observational study of upper gastrointestinal haemorrhage in elderly patients given selective cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs. BMJ 2002 Sep 21; 325(7365): 624
Bannwarth B. Risk-benefit assessment of opioids in chronic noncancer pain. Drug Saf 1999 Oct; 21(4): 283–96
Kalso E, Edwards JE, Moore RA, et al. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain 2004 Dec; 112(3): 372–80
Furlan AD, Sandoval JA, Mailis-Gagnon A,et al. Opioids for chronic noncancer pain: a meta-analysis of effectiveness and side effects. CMAJ 2006 May 23; 174(11): 1589–94
Kivitz A, Ma C, Ahdieh H, et al. A 2-week, multicenter, randomized, double-blind, placebo-controlled, dose-ranging, phase III trial comparing the efficacy of oxymorphone extended release and placebo in adults with pain associated with osteoarthritis of the hip or knee. Clin Ther 2006 Mar; 28(3): 352–64
Markenson JA, Croft J, Zhang PG, et al. Treatment of persistent pain associated with osteoarthritis with controlled-release oxycodone tablets in a randomized controlled clinical trial. Clin J Pain 2005 Nov; 21(6): 524–35
Portenoy RK. Opioid therapy for chronic nonmalignant pain: a review of the critical issues. J Pain Symptom Manage 1996 Apr; 11(4): 203–17
Christo PJ. Opioid effectiveness and side effects in chronic pain. Anesthesiol Clin North America 2003 Dec; 21(4): 699–713
McNicol E, Horowicz-Mehler N, Fisk RA, et al. Management of opioid side effects in cancer-related and chronic noncancer pain: a systematic review. J Pain 2003 Jun; 4(5): 231–56
Trescot AM, Glaser SE, Hansen H, et al. Effectiveness of opioids in the treatment of chronic non-cancer pain. Pain Physician 2008 Mar; 11(2 Suppl.): S181–200
Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg 2001 Nov; 182(5A Suppl.): 11S–8S
Tzschentke TM, Christoph T, Kögel B, et al. (−)-(1R,2R)-3-(3-Dimethylamino-1-ethyl-2-methyl-propyl)-phenol hydrochloride (tapentadol HCl): a novel μ-opioid receptor agonist/ norepinephrine reuptake inhibitor with broad-spectrum analgesic properties. J Pharmacol Exp Ther 2007 Oct; 323(1): 265–76
Carter GT, Sullivan MD. Antidepressants in pain management. Curr Opin Investig Drugs 2002 Mar; 3(3): 454–8
Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med 1992 May 7; 326(19): 1250–6
Ossipov MH, Malseed RT, Goldstein FJ. Augmentation of central and peripheral morphine analgesia by desipramine. Arch Int Pharmacodyn Ther 1982 Oct; 259(2): 222–9
Tzschentke TM, De Vry J, Terlinden R, et al. Tapentadol HCl. Drugs Future 2006; 31(12): 1053–61
Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 1986 Aug; 29(8): 1039–49
OXYCONTIN® (oxycodone HCl controlled-release) tablets [package insert]. Stamford (CT): Purdue Pharma L.P., 2007
Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988 Dec; 15(12): 1833–40
Farrar JT, Young Jr JP, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001 Nov; 94(2): 149–58
Brooks R. EuroQol: the current state of play. Health Policy 1996 Jul; 37(1): 53–72
Ware Jr JE, Snow KK, Kosinski M, et al. SF-36 health survey manual and interpretation guide. Boston (MA): The Health Institute, New England Medical Center, 1993
Slappendel R, Simpson K, Dubois D, et al. Validation of the PAC-SYM questionnaire for opioid-induced constipation in patients with chronic low back pain. Eur J Pain 2006 Apr; 10(3): 209–17
Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs 2003 Apr; 35(2): 253–9
Handelsman L, Cochrane KJ, Aronson MJ, et al. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse 1987; 13(3): 293–308
Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008 Feb; 9(2): 105–21
Tzschentke TM, Jahnel U, Kogel B, et al. Tapentadol hydrochloride: a next-generation, centrally acting analgesic with two mechanisms of action in a single molecule. Drugs Today (Barc) 2009 Jul; 45(7): 483–96
Minami K, Uezono Y, Ueta Y. Pharmacological aspects of the effects of tramadol on G-protein coupled receptors. J Pharmacol Sci 2007 Mar; 103(3): 253–60
Hennies HH, Friderichs E, Schneider J. Receptor binding, analgesic and antitussive potency of tramadol and other selected opioids. Arzneimittelforschung 1988 Jul; 38(7): 877–80
Barann M, Urban B, Stamer U, et al. Effects of tramadol and O-demethyl-tramadol on human 5-HT reuptake carriers and human 5-HT3A receptors: a possible mechanism for tramadol-induced early emesis. Eur J Pharmacol 2006 Feb 15; 531(1–3): 54–8
Buynak R, Shapiro D, Okamoto A, et al. Efficacy and safety of tapentadol ER for chronic low back pain: results of a randomized, double-blind, placebo- and active-controlled phase III study [abstract no. 301]. J Pain 2009 Apr; 10(4 Suppl. 1): S50
Wild JE, Grond S, Kuperwasser B, et al. Long-term safety and tolerability of tapentadol extended release for the management of chronic low back pain or osteoarthritis pain. Pain Pract. In press
Babul N, Noveck R, Chipman H, et al. Efficacy and safety of extended-release, once-daily tramadol in chronic pain: a randomized 12-week clinical trial in osteoarthritis of the knee. J Pain Symptom Manage 2004 Jul; 28(1): 59–71
Pascual MLG, Fleming RRB, Gana TJ, et al. Open-label study of the safety and effectiveness of long-term therapy with extended-release tramadol in the management of chronic nonmalignant pain. Curr Med Res Opin 2007; 23(10): 2531–42
Vorsanger GJ, Xiang J, Gana TJ, et al. Extended-release tramadol (tramadol ER) in the treatment of chronic low back pain. J Opioid Manag 2008 Mar; 4(2): 87–97
Reeves RR, Burke RS. Tramadol: basic pharmacology and emerging concepts. Drugs Today (Barc) 2008 Nov; 44(11): 827–36
Acknowledgements
This study was funded by Johnson & Johnson Pharmaceutical Research & Development, L.L.C. M. Afilalo received funding for study support from Johnson & Johnson Pharmaceutical Research & Development, L.L.C. M. Etropolski, B. Kuperwasser, K. Kelly, A. Okamoto, I. Van Hove, C. Rauschkolb, and J. Haeussler are Johnson & Johnson employees and shareholders. A. Steup and B. Lange are employees of Grünenthal GmbH. Editorial support for the writing of this manuscript was provided by Cherie Koch, PhD, of MedErgy, and was funded by Johnson & Johnson Pharmaceutical Services, L.L.C., and Grünenthal GmbH. M. Afilalo, who is not employed by Johnson & Johnson Pharmaceutical Services, L.L.C., or Grünenthal GmbH, was not compensated for his contribution to the writing of this manuscript and has no conflicts of interest that are directly relevant to the content of this study. Although writing assistance was provided, the authors retained full editorial control over the content of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Afilalo, M., Etropolski, M.S., Kuperwasser, B. et al. Efficacy and Safety of Tapentadol Extended Release Compared with Oxycodone Controlled Release for the Management of Moderate to Severe Chronic Pain Related to Osteoarthritis of the Knee. Clin. Drug Investig. 30, 489–505 (2010). https://doi.org/10.2165/11533440-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11533440-000000000-00000



