Abstract
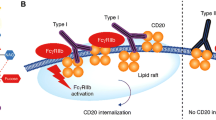
Ofatumumab is a fully human anti-CD20 monoclonal antibody that induces antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity in CD20-expressing B lymphocytes. Ofatumumab is highly potent in lysing B cells, and this appears to stem from its binding site on the short extracellular loop of the target CD20 protein and its slow release from the target molecule.
In a pivotal, noncomparative study in patients with fludarabine- and alemtuzumab-refractory chronic lymphocytic leukaemia (CLL), ofatumumab induced objective responses in 58% (99% CI 40, 74) of patients, which met a prespecified superiority criterion. The median duration of response was 7.1 months.
The median progression-free survival was 5.7 months and the median overall survival was 13.7 months.
In patients with fludarabine- and alemtuzumab-refractory CLL, infections and neutropenia were the most frequent treatment-related adverse events (all grades) that occurred during ofatumumab treatment; infections that commenced during treatment led to death in five patients (8%).

Similar content being viewed by others
References
Montserrat E, Moreno C. Chronic lymphocytic leukaemia: a short overview. Ann Oncol 2008 Sep; 19 Suppl. 7: vii320–5
Shanafelt TD, Call TG. Current approach to diagnosis and management of chronic lymphocytic leukemia. Mayo Clin Proc 2004 Mar; 79(3): 388–98
Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008 Jun 15; 111(12): 5446–56
Faderi S, Ferrajoli A, Frankfurt O, et al. Treatment of B-cell chronic lymphocytic leukemia with nonchemotherapeutic agents: experience with single-agent and combination therapy. Leukemia 2009; 23(3): 457–66
Tsimberidou A-M, Keating MJ. Treatment of fludarabine-refractory chronic lymphocytic leukemia. Cancer 2009 Jul 1; 115:2824–36
Tam CS, O’Brien S, Lerner S, et al. The natural history of fludarabine-refractory chronic lymphocytic leukemia patients who fail alemtuzumab or have bulky lymphade-nopathy. Leuk Lymphoma 2007 Oct; 48(10): 1931–9
GlaxoSmithKline. Arzerra™: prescribing information [online]. Available from URL: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/125326lbl.pdf [Accessed 2010 Jan 25]
Genmab. GlaxoSmithKline receives conditional marketing authorization in the EU for Arzerra™ (ofatumumab) [media release]. 2010 Apr 19
Bleeker WK, Munk ME, Mackus WJ, et al. Estimation of dose requirements for sustained in vivo activity of a therapeutic human anti-CD20 antibody. Br J Haematol 2008 Feb; 140(3): 303–12
Teeling JL, Mackus WJ, Wiegman LJ, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD 20. J Immunol 2006 Jul 1; 177(1): 362–71
Pawluczkowycz AW, Beurskens FJ, Beum PV, et al. Binding of submaximal C1q promotes complement-dependent cytotoxicity (CDC) of B cells opsonized with anti-CD20 mAbs ofatumumab (OFA) or rituximab (RTX): considerably higher levels of CDC are induced by OFA than by RTX. J Immunol 2009 Jul 1; 183(1): 749–58
Beum PV, Lindorfer MA, Beurskens F, et al. Complement activation on B lymphocytes opsonized with rituximab or ofatumumab produces substantial changes in membrane structure preceding cell lysis. J Immunol 2008 Jul 1; 181(1): 822–32
Craigen JL, Mackus WJM, Engleberts P, et al. Ofatumumab, a human Mab targeting a membrane-proximal small-loop epitope on CD20, induces potent NK cell-mediated ADCC [abstract no. 1725]. 51st Annual Meeting and Exposition of the American Society of Hematology; 2009 Dec 5–8; New Orleans (LA)
GlaxoSmithKline. Arzerra™ (ofatumumab) injection for intravenous use: FDA Oncological Drugs Advisory Committee briefing document [online]. Available from URL: http://www.fda.gov/ohrms/dockets/ac/09/briefing/2009-4444b1-02-GSK.pdf [Accessed 2010 Jan 26]
Osterborg A, Biilmann Ronn B, Jewell RC, et al. Correlation between serum ofatumumab concentrations, baseline patient characteristics and clinical outcomes in patients with fludarabine-refractory chronic lymphocytic leukemia (CLL) treated with single-agent ofatumumab [abstract no. 3433]. 51st Annual Meeting and Exposition of the American Society of Hematology; 2009 Dec 5–8; New Orleans (LA)
Wierda WG, Kipps TJ, Mayer J, et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol 2010 Apr 1; 28(10): 1749–55
Coiffier B, Lepretre S, Pedersen LM, et al. Safety and efficacy of ofatumumab, a fully human monoclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: a phase 1–2 study. Blood 2008 Feb 1; 111(3): 1094–100
National Cancer Institute. Common terminology criteria [online]. Available from URL: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf[Accessed2010Feb3]
European Medicines Agency. Arzerra: summary of opinion (initial authorisation) [online]. Available from URL: http://www.emea.europa.eu/pdfs/human/opinion/Arzerra_214261en.pdf [Accessed 2010 Jan 25]
Acknowledgements and Disclosures
The manuscript was reviewed by: C. Pepper, Department of Haematology, School of Medicine, Cardiff University, Cardiff, UK; E. Terpos, Department of Clinical Therapeutics, University of Athens School of Medicine, Athens, Greece.
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturers of the agent under review were offered an opportunity to comment on this article; changes based on any comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sanford, M., McCormack, P.L. Ofatumumab. Drugs 70, 1013–1019 (2010). https://doi.org/10.2165/11203850-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11203850-000000000-00000