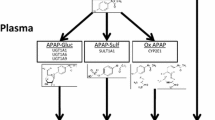
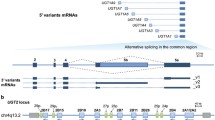
Abstract
The uridine diphosphate (UDP)-glucuronosyltransferases (UGTs) are key enzymes in human detoxication of xeno- and endobiotics. Potentially toxic endogenous compounds such as bilirubin, or exogenous compounds such as drugs, pesticides, and carcinogens, are generally transformed into water-soluble glucuronides for excretion in bile and urine.
The UGTs are encoded by a multigene family in humans. A relatively small number of human enzymes catalyze the glucuronidation of thousands of compounds. Genetic variations and single nucleotide polymorphisms (SNPs) within the UGT genes are remarkably common, and lead to genetic polymorphisms. The multiplicity of transferases, some exhibiting overlapping substrate specificity, may provide functional compensation for genetic deficit in some cases. Genetic variation may cause different phenotypes by affecting expression levels or activities of individual UGTs. This inter-individual variation in UGTs has resulted in functional deficit affecting endogenous metabolism and leading to jaundice and other diseases. Disruption of the normal metabolic physiology, by the reduction of bile acid excretion or steroid glucuronidation, may lead to cholestasis and organ dysfunction. Deficient glucuronidation of drugs and xenobiotics have an important pharmacological impact, which may lead to drug-induced adverse reactions, and even cancer. Additional novel polymorphisms in this gene family are yet to be revealed and studied, but will have a profound effect on the development of new drugs and therapies.











Similar content being viewed by others
References
Dutton GJ, editor. Glucuronidation of drugs and other compounds. Boca Raton (FL): CRC Press, 1980
Clarke DJ, Burchell B. The uridine diphosphate glucuronosyltransferase multigene family: function and regulation. Handb Exper Pharmcol 1994; 112: 3–43
Burchell B, McGurk K, Brierley CH, et al. UDP-glucuronosyltransferases. In: Sipes IG, Gandolfi AJ, Mcqueen CA, editors. Comprehensive toxicology. Vol. 3. Amsterdam: Pergamon Elsevier Science, 1997: 401–35
Brierley CH, Burchell B. Human UDP-glucuronosyltransferases: chemical defence, jaundice and gene therapy. Bioessays 1993; 15: 749–54
Spahn-Langguth H, Benet LZ. Acyl glucuronides revisited: is the glucuronidation process a toxification as well as detoxification mechanism? Drug Metab Rev 1992; 24: 5–48
Burchell B, Brierley CH, Rance D. Specificity of human UDP-glucuronosyltransferases and xenobiotic glucuronidation. Life Sci 1995; 57: 1819–31
Remmel RP, Burchell B. Validation and use of cloned, expressed human drug metabolising enzymes in heterologous cells for analysis of drug metabolism and drug-drug interactions. Biochem Pharmacol 1993; 46: 559–66
Tukey RH, Strassburg CP. Genetic multiplicity of the human UDP-glucuronosyltransferases and regulation in the gastrointestinal tract. Mol Pharmacol 2001; 59: 405–14
Radominska-Pandya A, Czernik PJ, Little JM, et al. Structural and functional studies of UDP-Glucuronosyltransferases. Drug Metab Rev 1999; 31: 817–99
Burchell B, Nebert DW, Nelson DR, et al. The UDP glucuronosyltransferase gene superfamily: suggested nomenclature based on evolutionary divergence. DNA Cell Biol 1991; 10: 487–94
Mackenzie P, Owens IS, Burchell B, et al. The UDP glycosyltransferase gene superfamily: recommended nomenclature update based on evolutionary divergence. Pharmacogenetics 1997; 7: 255–69
Moghrabi N, Sutherland L, Wooster R, et al. Chromosomal assignment of human phenol and bilirubin UDP-glucuronosyltransferase genes (UGT1A Subfamily). Ann Hum Genet 1992; 56: 83–93
Owens IS, Ritter JK. The novel bilirubin/phenol UDP-glucuronosyltransferase UGT 1 gene locus: implications for multiple nonhemolytic familial hyperbilirubinaemia phenotypes. Pharmacogenetics 1992; 2: 93–108
Wooster R, Sutherland L, Ebner T, et al. Cloning and stable expression of a new member of the human liver phenol/bilirubin UDP-glucuronosyltransferase cDNA family. Biochem J 1991; 278: 465–9
Gong Q-H, Cho JW, Huang T, et al. Thirteen UDP-glucuronosyltransferase genes are encoded at the human UGT1 gene complex locus. Pharmacogenetics 2001; 11: 357–68
Pacifici GM, Pelkonen O, editors. Interindividual variability in human drug metabolism. London: Taylor & Francis, 2001
Monaghan G, Povey S, Burchell B, et al. Localization of a bile acid UDP-glucuronosyltransferase gene UGT2B to chromosome 4 using the polymerase chain reaction. Genomics 1992; 13: 908–9
Monaghan G, Clarke DJ, Povey S, et al. Isolation of a human YAC contig encompassing a cluster of UGT2 genes and its regional localization to chromosome 4q13. Genomics 1994; 23: 496–9
Reidy M, Wang J-Y, Miller AP, et al. Genomic organisation of the UGT2B gene cluster on human chromosome 4q13. Pharmacogenetics 2000; 10: 251–60
Turgeon D, Carrier J-S, Levesque E, et al. Relative enzymatic activity, protein stability, and tissue distribution of human steroid-metabolizing UGT2B subfamily members. Endocrinology 2001; 142: 778–87
Jedlitschky G, Cassidy AJ, Sales M, et al. Cloning and characterization of a novel human olfactory UGT. Biochem J 1999; 340: 837–43
Tephly TR, Burchell B. The UDP-glucuronosyltransferases: afamily of detoxifying enzymes. Trends Pharmacol Sci 1990; 11: 276–9
Senafi SB, Clarke DJ, Burchell B. Investigation of the substrate-specificity of a cloned expressed human bilirubin UDP-glucuronosyltransferase: UDP-sugar specificity and involvement in steroid and xenobiotic glucuronidation. Biochem J 1994; 303: 233–40
Temellini A, Giuliani L, Pacifici GM. Interindividual variability in the glucuronidation and sulphation of ethinyloestradiol in human liver. Br J Clin Pharmacol 1991; 31: 661–4
Soars MG, Riley RJ, Findlay KAB, et al. Evidence for significant differences in microsomal drug glucuronidation by canine and human liver and kidney. Drug Metab Dispos 2001; 29: 121–6
Burchell B, Coughtrie MWH. UDP-glucuronyltransferases in “genetic factors influencing the metabolism of foreign compounds”. Pharmacol Ther 1989; 43: 261–89
Vainio H, Elovaara E, Luukkanen L, et al. Expression and co-induction of CYP1A1 and UGT1*6 in human lungs. Eur J Drug Metab Pharmacokinet 1995; Special Issue: 47–8
Benowitz NL, Perez-Stable E, Fong I, et al. Ethnic differences in N-glucuronidation of nicotine and cotinine. J Pharmacol Exp Ther 1999; 291: 1196–203
Shimoda K, Noguchi T, Ozeki Y, et al. Metabolism of clomipramine in a Japanese psychiatric population: hydroxylation, desmethylation, and glucuronidation. Neuropsychopharmacology 1995; 12: 323–33
Bock KW, Schrenk D, Forster A, et al. The influence of environmental and genetic factors on CYP2D6, CYP1A2 and UDP-glucuronosyltransferases in man using sparteine, caffeine, and paracetamol as probes. Pharmacogenetics 1994; 4: 209–18
Liu HF, Vincentviry M, Galteau MM, et al. Urinary glucuronide excretion of fenofibric and clofibric acid glucuronides in man: is it polymorphic. Eur J Clin Pharmacol 1991; 41: 153–9
Vincent-Viry M, Cossy C, Galteau MM, et al. Lack of a genetic polymorphism in the glucuronidation of fenofibric acid. Pharmacogenetics 1995; 5: 50–2
Duche JC, Querol-Ferrer V, Barre J, et al. Dextromethorphan O-demethylation and dextrorphan glucuronidation in a French population. Int J Clin Pharmacol Ther Toxicol 1993; 31: 392–8
Fischer D, Breyer-Pfaff U. Variability of diphenhydramine N-glucuronidation in healthy subjects. Eur J Drug Metab Pharmacokinet 1997; 22: 151–4
Sherwood RA, Marsden JT, Stein CA, et al. Intra-individual variation in serum AZT is not related to intestinal absorption or small intestinal inflammatory changes in human HIV-infected subjects. Antivir Chem Chemother 1997; 8: 327–32
Bowman ED, Rothman N, Hackl C, et al. Interindividual variation in the levels of certain urinary polycyclic aromatic hydrocarbon metabolites following medicinal exposure to coal tar ointment. Biomarkers 1997; 2: 321–7
Court MH, Duan SX, Van Moltke LL, et al. Interindividual variability in acetaminophen glucuronidation by human liver microsomes: identification of relevant acetaminophen UDP-glucuronosyltransferase isoforms. J Pharmacol Exp Ther 2001; 299: 988–1006
Toide K, Takahashi Y, Yamazaki H, et al. Hepatocyte nuclear factor-1α is a causal factor responsible for interindividual differences in the expression of UDP-Glucuronosyltransferase 2B7 mRNA in human livers. Drug Metab Dispos 2002; 30: 613–5
Brierley CH, Senafi SB, Clarke D, et al. Regulation of the human bilirubin UDP-Glucuronosyltransferase gene. Adv Enzyme Regul 1996; 36: 85–97
Lee Y-H, Sauer B, Johnson PF, et al. Disruption of the c/ebpα gene in adult mouse liver. Mol Cell Biol 1997; 17: 6014–22
Bosma PJ, Roy Chowdhury J, Bakker C, et al. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. N Engl J Med 1995; 333: 1171–218
Monaghan G, Ryan MF, et al. Genetic variation in bilirubin UDP-glucuronosyltransferase gene promoter and Gilbert’s syndrome. Lancet 1996; 347: 578–81
Sugatani J, Yamakawa K, Yoshinari K, et al. Identification of a defect in the UGT1A1 gene promoter and its association with hyperbilirubinaemia. Biochem Biophys Res Commun 2002; 292: 492–7
Innocenti F, Grimsley C, Das S, et al. Haplotype structure of the UDP-glucuronosyltransferase 1A1 promoter in different ethnic groups. Pharmacogenetics 2002; 12: 725–33
Patel M, Tang BK, Grant DM, et al. Interindividual variability in the glucuronidation of (S) oxazepam contrasted with that of (R) oxazepam. Pharmacogenetics 1995; 5: 287–97
Bhasker CR, McKinnon W, Stone A, et al. Genetic polymorphism of UDP-glucuronosyltransferase 2B7 (UGT2B7) at amino acid 268: ethnic diversity of alleles and potential clinical significance. Pharmacogenetics 2000; 10: 679–85
Koiwai O, Nishizawa M, Hasada K, et al. Gilbert’s syndrome is caused by heterozygous missense mutation in the gene for bilirubin UDP-glucuronosyltransferase. Hum Mol Genet 1995; 4: 1183–6
Ciotti M, Marrone A, Potter C, et al. Genetic polymorphism in the human UGT1A6 (planar phenol) UDP-glucuronosyltransferase: pharmacological implications. Pharmacogenetics 1997; 7: 485–95
Guillemette C, Ritter JK, Auyeung DJ, et al. Structural heterogeneity at the UDP-glucuronosyltransferase 1 locus: functional consequences of three novel missense mutations in the human UGT1A7 gene. Pharmacogenetics 2000; 10: 629–44
Vogel A, Kneip S, Barut A, et al. Genetic link of hepatocellular carcinoma with polymorphisms of the UDP-glucuronosyltransferase UGT1A7 gene. Gastroenterology 2001; 121: 1136–44
Zheng Z, Guillemette C, Park JY, et al. The tobacco carcinogen-detoxifying enzyme UGT1A7 and its association with oralaryngeal cancer risk in Caucasian and African Americans. J Natl Cancer Inst 2001; 29: 1343–8
Huang Y-H, Galijatovic A, Nguyen N, et al. Identification and functional characterization of UDP-glucuronosyltransferases UGT1A8*1, UGT1A8*2 and UGT1A8*3. Pharmacogenetics 2002; 12: 287–97
Levesque E, Beaulieu M, Hum D, et al. Characterization and substrate specificity of UGT2B4 (E458), a UDP-Glucuronosyltransferase encoded by a polymorphic gene. Pharmacogenetics 1999; 9: 207–16
Court MH, Duan SX, Guillemette C, et al. Stereoselective conjugation of oxazepam by human UDP-glucuronosyltransferases (UGTs): S-oxazepam is glucuronidated by UGT2B15, while R-oxazepam is glucuronidated by UGT2B7 and UGT1A9. Drug Metab Dispos 2002; 30: 1257–65
Levesque E, Beaulieu M, Green MD, et al. T1: islation and characterization of UGT2B15(Y-85): A UDP-glucuronosyltransferase encoded by a polymorphic gene. Pharmacogenetics 1997; 7: 317–25
Clarke DJ, Moghrabi N, Monaghan G, et al. Genetic defects of the UDP-glucuronosyltransferase-1 (UGT1) gene that cause familial non-haemolytic unconjugated hyperbilirubinaemias. Clin Chim Acta 1997; 266: 63–74
Arias IM. Chronic unconjugated hyperbilirubinaemia without overt signs of hemolysis in adolescents and adults. J Clin Invest 1962; 41: 2233–45
Szabo L, Ebrey P. Studies on the inheritance of Crigler-Najjar syndrome by the menthol test. Acta Paediatr Scand 1963; 4: 153–8
Arias IM, Gartner LM, Cohen M, et al. Chronic nonhemolytic unconjugated hyperbilirubinemia with glucuronyltransferase deficiency: clinical, biochemical, pharmacologic and genetic evidence for heterogeneity. Am J Med 1969; 47: 395–409
Bloomer JR, Berk PD, Howe RB, et al. Bilirubin metabolism in congenital nonhemolytic jaundice. Pediatr Res 1971; 5: 256–64
Findlay K, Burchell B. Characterization of the UGTs catalysing thyroid hormone glucuronidation in man. J Clin Endocrinol 2000; 85: 2879–83
King CD, Green MD, Rios GR, et al. The glucuronidation of exogenous and endogenous compounds by stably expressed rat and human UDP-glucuronosyltransferase 1.1. Arch Biochem Biophys 1996; 332: 92–100
Ebner T, Burchell B, Remmel RP. Human bilirubin UDP-glucuronosyltransferase catalyses the glucuronidation of ethinylestradiol. Mol Pharmacol 1993; 43: 649–54
Iyer L, King CD, Whitington PF, et al. Genetic predisposition to the metabolism of irinotecan (CPT11). J Clin Invest 1998; 101: 847–54
Moghrabi N, Clarke DJ, Burchell B, et al. Cosegration of intragenic markers with a novel mutation that causes Crigler-Najjar Syndrome type 1: implications for carrier detection and prenatal diagnosis. Am J Hum Genet 1993; 53: 722–9
Fevery J. Pathogenesis of Gilbert’s syndrome. Eur J Clin Invest 1981; 11: 417–8
Macklon AF, Savage RL, Rawlins MD. Gilbert’s syndrome and drug metabolism. Clin Pharmacokinet 1979; 4: 223–32
Ullrich D, Sieg A, Blume R, et al. Normal pathways for glucuronidation, sulphation and oxidation of paracetamol in Gilbert’s syndrome. Eur J Clin Invest 1987; 17: 237–40
De Morais SMF, Uetrecht JP, Wells PG. Decreased glucuronidation and increased bioactivation of acetaminophen in Gilbert’s syndrome. Gastroenterology 1992; 102: 577–86
Raijmakers MT, Jansen PL, Steegers EA, et al. Association of human liver bilirubin UDP-glucuronosyltransferase activity with a polymorphism in the promoter region of the UGT1A1 gene. J Hepatol 2000; 33: 348–51
Monaghan G, Foster B, Jurima-Romet M, et al. UGT1*1 genotyping in a Canadian inuit population. Pharmacogenetics 1997; 7: 153–6
Balram C, Sabapathy K, Fei G, et al. Genetic polymorphisms of UDP-glucuronosyltransferase in Asians: UGT1A1*28 is a common allele in Indians. Pharmacogenetics 2002; 12: 81–3
Sampietro M, Lupica L, Perrero L, et al. TATA-box promoter mutant in the promoter of UDP-glucuronosyltransferase gene in Italian patients with Gilbert’s syndrome. Ital J Gastroenterol Hepatol 1998; 30: 194–8
Biondi ML, Turri O, Dilillo E, et al. Contribution of the TATA-Box genotype (Gilbert’s syndrome) to serum bilirubin concentrations in the Italian population. Clin Chem 1999; 45: 897–8
Beutler E, Gelbert T, Demina A, et al. Racial variability in the UDP-glucuronosyltransferase 1 (UGT1A1) promoter: a balanced polymorphism for regulation of bilirubin metabolism. Proc Natl Acad Sci U S A 1998; 95: 8170–4
te Morsche RHM, Zusterzeel PLM, Raijmakers MTM, et al. Polymorphism in the promoter region of the bilirubin UDP-glucuronosyltransferase (Gilbert’s syndrome) in healthy Dutch subjects [letter]. Hepatology 2001; 33: 765
Borlak J, Thum T, Landt O, et al. Molecular diagnosis of a familial nonhemolytic hyperbilirubinaemia (Gilbert healthy subjects). Hepatology 2000; 32: 792–5
Ando Y, Chida M, Nakayama K, et al. The UGT1A1*28 allele is relatively rare in a Japanese population. Pharmacogenet 1998; 8: 357–60
Sato H, Adachi Y, Koiwai O. The genetic basis of Gilbert’s syndrome. Lancet 1996; 347: 557–8
Akaba K, Kimura T, Sasaki A, et al. Neonatal hyperbilirubinaemia and mutation of bilirubin UDP-glucuronosyltransferase gene: a common missense mutation among Japanese, Koreans and Chinese. Biochem Mol Biol Int 1998; 46: 21–6
Yamamoto K, Sato H, Fujiyama Y, et al. Contribution of two missense mutation (G71R and Y486D) of the bilirubin UDP-glucuronosyltransferase (UGT1A1) gene to phenotypes of Gilbert’s syndrome and Crigler-Najjar syndrome type II. Biochim Biophys Acta 1998; 1406: 267–73
Aono S, Adachi Y, Uyama E, et al. Analysis of genes for bilirubin UDP-glucuronosyltransferase in Gilbert’s syndrome. Lancet 1995; 345: 958–9
Huang C-S, Luo G-A, Huang M-H, et al. Variations of the bilirubin in uridine-diphosphoglucuronosyltransferase 1A1 gene in healthy Taiwanese. Pharmacogenetics 2000; 10: 539–44
Ciotti M, Owens IS. Evidence for overlapping active sites for 17α-Ethinylestradiol and bilirubin in the human major bilirubin UDPglucuronosyltransferase. Biochem 1996; 35: 10119–24
Fisher MB, Vanden Branden M, Findlay K, et al. Tissue distribution and interindividual variation in human UDP-glucuronosyltransferase activity: relationship between UGT1A1 promoter genotype and variability in a liver bank. Pharmacogenetics 2000; 10: 727–39
Wasserman E, Myara A, Lokiec F, et al. Severe CPT-11 toxicity in patients with Gilbert’s syndrome: two case reports. Ann Oncol 1997; 810: 1049–51
Gupta E, Mick R, Ramirez J, et al. Pharmacokinetic and pharmacodynamic evaluation of the topoisomerase inhibitor irinotecan in cancer patients. J Clin Oncol 1997; 15: 1502–10
Saliba F, Higipantelli R, Misset JL, et al. Pathophysiology and therapy of irinotecaninduced onset diarrhoea in patients with advanced colorectal cancer. J Clin Oncol 1998; 16: 2745–51
Ando Y, Saka H, Asai G, et al. UGT1A1 genotypes and glucuronidation of SN-38, the active metabolite of irinotecan. Ann Oncol 1998; 9: 845–7
Iyer L, Hall D, Das S, et al. Phenotype-genotype correlation of in vitro SN-38 (active metabolite of irinotecan) and bilirubin glucuronidation in human liver tissue with UGT1A1 promoter polymorphism. Clin Pharmacol Ther 1999; 65: 576–82
Iyer L, Das S, Janisch L, et al. UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenomics J 2002; 2: 43–7
Ando Y, Ueoka H, Sugiyama T, et al. Polymorphisms of UDP-glucuronosyltransferase and pharmacokinetics of irinotecan. Ther Drug Monit 2002; 24: 111–6
Ciotti M, Basu N, Brangi M, et al. Glucuronidation of 7-Ethyl-10-hydroxycamptothecin (SN38) by the human UDP-glucuronosyltransferases encoded at the UGT1 locus. Biochem Biophys Res Commun 1999; 260: 199–202
Hanioka N, Ozawa S, Jinno H, et al. Human liver UDP-glucuronosyltransferase isoforms involved in the glucuronidfation of 7-ethyl-10-hydroxycamptothecin. Xenobiotica 2001; 31: 687–99
Takasuna K, Hagiwara T, Hirohashi M, et al. Inhibition of intestinal microflora beta-glucuronidase modifes the distribution of the active metabolite of the anti-tumor agent, irinotecan hydrochloride (CPT-11) in rats. Cancer Chemother Pharmacol 1998; 42: 280–6
Maruo Y, Sata H, Bamba N, et al. Chemotherapy-induced unconjugated hyperbilirubinaemia caused by a mutation of the bilirubin uridine-5′-diphosphate glucuronosyltransferase gene. J Pediatr Hematol Oncol 2001; 23: 45–7
O’Mara EM, Mummanemi V, Burchell B, et al. Relationship between UDP-glucuronosyltransferase 1A1 genotype and total bilirubin elevation in healthy subjects receiving BMS-232632 and saquinavir [abstract]. 40th ICAAC Meeting; 2000 Sep 17–21, Toronto, Canada
Zucker SD, Qin X, Rouster SD, et al. Mechanism of indinavir-induced hyperbilirubinaemia. Proc Natl Acad Sci (USA) 2001; 98: 12671–6
Herman RJ, Chaudhary A, Szakacs CB. Disposition of Lorazepam in Gilbert’s Syndrome: effects of fasting, feeding, and enterohepatic circulation. J Clin Pharmacol 1994; 34: 978–84
Chowdhury RJ, Wolkoff A, Chowdhury RN, et al. Hereditary jaundice and disorders of bilirubin metabolism. In: Scriver CR, Beaudet A, Sly W, et al., editors. The metabolic basis of inherited disease. New York: McGraw-Hill, 1995: 2161–208
Onishi S, Kawade N, Itoh S, et al. Postnatal development of uridine, diphosphate glucuronosyltransferase activity towads bilirubin and 2-aminophenol in human liver. Biochem J 1979; 184: 705–7
Arias IM, Gartner LM, Seifter S, et al. Prolonged neonatal unconjugated hyperbilirubinaemia associated with breast-feeding and a steroid, pregnane-3α,20β-diol in maternal milk that inhibits glucuronide formation in vitro. J Clin Invest 1964; 43: 2037–47
Winfield CR, MacFaul R. Clinical study of prolonged jaundice in breast and bottle-fed babies. Arch Dis Child 1978; 54: 506–7
Osborn LM, Reiff M, Bolus R. Jaundice in the full-term neonate. Pediatrics 1984; 73: 520–5
Maisels MK, Gifford K, Antle CE, et al. Jaundice in the healthy newborn infant: a new approach to an old problem. Pediatrics 1988; 81: 505–11
Maisels MJ, Gifford K. Neonatal jaundice in full-term infants: role of breast-feeding and other causes. Am J Dis Child 1983; 137: 561–2
Bancroft JD, Kreamer B, Gourley GR. Gilbert’s syndrome accelerates development of neonatal jaundice. J Paediatr 1998; 132: 656–60
Monaghan G, McLellan A, McGeechan A, et al. Gilbert’s syndrome is a contributory factor in very prolonged unconjugated hyperbilirubinaema of the newborn. J Pediatr 1999; 134: 441–6
Saland J, McNamara H, Cohen MI. Navajo jaundice: a variant of neonatal hyperbilirubinaemia associated with breast-feeding. J Pediatr 1974; 85: 271–5
Fisher Q, Cohen MI, Curda L. Jaundice and breast-feeding among Alaska Eskimo newborns. Am J Dis Child 1978; 132: 859–63
Johnson JD. Jaundice in Navajo neonates. Clin Paediatr (Phila) 1992; 12: 716–8
Kaplan M, Hammerman C. Severe neonatal hyperbilirubinaemia: a potential complication of glucose-6-phosphate dehydrogenase deficiency. Clin Perinatol 1998; 25: 575–92
Kaplan M, Renbaum P, Levy-Lahad E, et al. Gilbert’s syndrome and glucose-6-phosphate dehydrogenase deficiency: a dose-dependent genetic interaction crucial to neonatal hyperbilirubinaemia. Proc Natl Acad Sci U S A 1997; 94: 12128–32
Sanpietro M, Lupica L, Perrero L, et al. The expression of UDP-glucuronosyltransferase is a major determinant of bilirubin level in heterozygous β-thalassaemia and in glucose-6-phosphate dehydrogenase deficiency. Br J Haematol 1997; 99: 437–9
Gollanello R, Cipollino MD, Carboni G, et al. Hyperbilirubinaemia, glucose-6-phosphate dehydrogenase deficiency and Gilbert’s syndrome. Eur J Pediatr 1999; 158: 914–6
Kaplan M, Hammerman C, Renbaum P, et al. Gilbert’s syndrome and hyperbilirubinaemia in ABO-incompatible neonates. Lancet 2000; 356: 652–3
del Guidice EM, Perotta S, Nobili B, et al. Coinheritance of Gilbert’s syndrome increases the risk of developing gallstones in patients with hereditary spherocytosis. Blood 1999; 94: 2259–62
Perrotta S, del Giudice EM, Carbane R, et al. Gilbert’s syndrome accounts for the phenotypic variability of congenital dyserthropoietic anaemia type II. J Pediatr 2000; 136: 556–9
Kaplan M, Hammerman C, Rubaltelli FF, et al. Hemolysis and bilirubin conjugation in association with UDP-glucuronosyltransferase 1A1 promoter polymorphism. Hepatology 2002; 35: 905–11
Trioche P, Chalas J, Francoual J, et al. Jaundice with hypertrophie pyloric stenosis as an early manifestation of Gilbert’s Syndrome. Arch Dis Child 1999; 81: 301–3
Galanello R, Cipollina MD, Dessi C, et al. Co-inherited Gilbert’s syndrome: a factor determining hyperbilirubinaemia in homozygous beta-thalassemia. Haematologica 1999; 84: 103–5
McKie K, Kutlar F, Sromek S, et al. UDP-glucuronosyltransferase (UGT1A1) promoter polymorphism and bilirubin levels in patients with sickle cell disease [abstract 861]. Blood 1999; 94: P197a
Passon RG, Howard TA, Zimmerman SA, et al. The effect of UDP-glucuronosyltransferase (UGT1A1) promoter polymorphisms on serum bilirubin levels and cholelthiasis in patients with sickle cell anaemia [abstract 2865]. Blood 1999; 94: P645a
Doyama H, Okada T, Kobayashi T, et al. Effect of bilirubin UDP-glucuronosyltransferase 1 gene TATA box genotypes on serum bilirubin concentrations in chronic liver injuries. Hepatology 2000; 32: 563–8
Guillemette C, Millikan RC, Newman B, et al. Genetic polymorphisms in uridine diphospho-glucuronosyltransferase 1A1 and association with breast cancer among African Americans. Cancer Res 2000; 60: 950–6
Bock KW, Forster A, Gschaidmeier H, et al. Paracetamol glucuronidation by recombinant rat and human phenol UDP-glucuronosyltransferase. Biochem Pharmacol 1993; 45: 1809–14
Osborne NJ, Tonkin AL, Miners JO. Interethnic differences in drug glucuronidation: a comparison of paracetamol metabolism in Caucasians and Chinese. Br J Clin Pharmacol 1991; 32: 765–7
Ebner T, Burchell B. Substrate specificities of two stably expressed human liver UDP-glucuronosyltransferases of the UGT1 gene family. Drug Metab Dispos 1993; 21: 50–5
Cheng Z, Radominska-Paydya A, Tephly TR. Cloning and expression of human UDP-glucuronosyltransferase 1A8. Arch Biophys Biochem 1998; 356: 301–5
Lautala P, Ethell B, Burchell B, et al. The COMT inhibitors and entacapone and tolcapone are good substrates for UGT1A4 and UGT1A9. Drug Metab Dispos 2000; 28: 1385–9
Strassburg CP, Mann MP, Tukey RH. Expression of the UDP-glucuronosyltransferase 1A locus in human colon. J Biol Chem 1998; 273: 8719–26
Fournel-Gigleux S, Jackson MR, Wooster R, et al. Expression of a human liver cDNA encoding hydeoxycholic acid UDP-glucuronosyltransferase in cell culture. FEBS Lett 1989; 243: 119–22
Pillot T, Ouzzine M, Fournel-Gigleux S, et al. Glucuronidation of hyodeoxycholic acid in human liver: evidence for a selective role of UGT2B4. J Biol Chem 1993; 268: 25636–42
Jin CJ, Miners JO, Lillywhite KJ, et al. Complementary deoxyribonucleic acid cloning and expression of a human liver uridine diphosphate-glucuronosyltranferase glucuronidating carboxylic acid-containing drugs. J Pharmacol Exp Ther 1993; 264: 475–9
Ritter JK, Chen F, Sheen YY, et al. Two human liver cDNAs encode UDP-glucuronosyltransferase with 2 log differences in activity toward parallel substrates including hyodeoxycholic acid and certain estrogen derivatives. Biochem 1992; 31: 3409–14
Jin CJ, Mackenzie PI, Miners JO. The regio- and stereo-selectivity of C19 and C21 hydroxysteroid glucuronidation by UGT2B7 and UGT2B11. Arch Biochem Biophys 1997; 341: 207–11
Coffman BL, Rios GR, King CD, et al. Human UGT2B7 catalyses morphine glucuronidation. Drug Metab Dispos 1997; 25: 1–4
Patel M, Tang BK, Kalow W. (S) Oxazepam glucuronidation is inhibited by ketoprofen and other substrates of UGT 2B7. Pharmacogentics 1995; 5: 43–29
Coffman BL, King CD, Rios GR, et al. The glucuronidation of opioids, other xenobiotics and androgens by human UGT2B7Y(268) and UGT2B7H(268). Drug Metab Dispos 1998; 26: 73–7
Yue QY, Svensson JO, Alm C, et al. Interindividual and interethnic differences in the demethylation and glucuronidation of codeine. Br J Clin Pharmacol 1989; 28: 629–37
Yue Q-Y, Svensson J-O, Sawe J, et al. Codeine metabolism in three oriental populations: a pilot study in Chinese, Japanese and Koreans. Pharmacogenetics 1995; 5: 173–7
Green MD, Oturu EM, Tephly TR. Stable expression of a human liver UDP-glucuronosyltransferase (UGT2B15) with activity toward steroid and xenobiotic substrates. Drug Metab Dispos 1994; 22: 799–805
MacLeod SL, Nowell SA, Lang NP. Determination of genetic polymorphisms in UGT2B15 by allele-specific PCR. Ann Surg Oncol 2000; 7: 777–82
Lampe JW, Bigler J, Bush AC, et al. Prevalence of polymorphisms in the human UDP-glucuronosyltransferase 2B family: UGT2B4 ((DE)-E-458), UGT2B7 ((HY)-Y-268), and UGT2B15((DY)-Y-85). Cancer Epidemiol Biomarkers Prev 2000; 9: 329–33
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burchell, B. Genetic Variation of Human UDP-Glucuronosyltransferase. Am J Pharmacogenomics 3, 37–52 (2003). https://doi.org/10.2165/00129785-200303010-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00129785-200303010-00006