Summary
Abstract
The sirolimus-eluting stent (CYPHER™) is a metal stent coated with 140 μg/cm2 of sirolimus blended with synthetic polymers. After stent implantation, sirolimus is slowly released causing localized cytostatic inhibition of proliferation of vascular smooth muscle cells in the peri-stent arterial wall over a period of about 1 month. Only minimal amounts of sirolimus enter the bloodstream and these appear to be insufficient to be of clinical relevance.
In clinical trials that evaluated single de novo lesions and in-stent restenosis in patients with coronary artery disease, the sirolimus-eluting stent was associated with minimal neointimal hyperplasia. In four randomized trials in de novo lesions, sirolimus eluting stents produced a significantly lower incidence of major adverse cardiac events (MACE) than that observed in patients with uncoated stents, during periods of up to 2 years (p < 0.05 for all comparisons). The lower incidence of MACE was mostly due to a reduced requirement for repeat target vessel revascularization. At angiographic follow-up at periods of up to 8 months, late luminal loss was significantly smaller in the sirolimus-eluting stent groups than in the uncoated-stent groups (p < 0.001 in all studies).
The sirolimus-eluting stent was well tolerated in clinical trials of up to 3 years’ follow-up. Because minimal blood levels of sirolimus are achieved, systemic adverse effects appear to be avoided. To date, there has been no evidence of any potential adverse effects resulting from the polymer coating or local drug toxicity. As yet, there is no evidence of an increased risk of subacute or late thrombosis, or aneurysm formation with the sirolimus-eluting stent compared with the uncoated stent. Long-term follow-up is needed to fully assess these theoretical concerns. Cost-effectiveness analyses over 12 months demonstrated a cost advantage for the sirolimus-eluting stent compared with an uncoated stent because of a reduced requirement for repeat target vessel revascularizations.
Conclusion: Initial clinical trials with the sirolimus-eluting stent in patients with de novo coronary lesions have shown a significantly reduced incidence of MACE and of restenosis compared with a standard stent. Efficacy appears to be maintained throughout follow-up periods of up to 2 years in randomized trials, and to date, systemic or local adverse effects have been avoided. If efficacy and tolerability are consistently demonstrated over the long term, the sirolimus-eluting stent will be a major advance in the control of in-stent restenosis.
Pharmacodynamic Properties
Sirolimus contained in the sirolimus-eluting stent counteracts cytokine effects on cell proliferation and has a cytostatic effect on vascular smooth muscle cells. In vitro, sirolimus inhibits the proliferation of T- and B-lymphocytes, peripheral blood mononuclear cells, and mast cells. Sirolimus also inhibits in vitro vascular smooth muscle cell proliferation and migration stimulated by platelet-derived growth factor. It also markedly inhibited cultured endothelial cell growth, but in human vascular smooth muscle cells, sirolimus did not induce in vitro expression of plasminogen activator inhibitor-1.
In in vivo animal models, sirolimus-eluting stents compared with bare-metal control stents were associated with lower inflammation scores and limited peri-stent inflammatory cell infiltration, and with reduced expression of monocyte chemoattractant protein-1 and interleukin-6. Sirolimus-eluting stents markedly reduced neointimal area and mean percentage stenosis, relative to bare-metal or polymer-coated control stents, 28–30 days after stent implantation. In one study, these parameters were reduced only after mild rather than severe injury; in another, the reduction in neointimal area was not sustained beyond 30 days; whereas in another trial, the reduction was maintained at 180 days. Sirolimus-eluting stents also reduced intimal thickness and intima: media thickness relative to control stents; stents eluting sirolimus plus dexamethasone were no more effective than sirolimus-eluting stents, although both stents were significantly superior to dexamethasone-eluting stents; and sirolimus-eluting stents were also associated with significantly lower histological scores for intimal smooth muscle cell content, yet significantly greater scores for fibrin deposition, than bare-metal control stents. Moreover, adequate endothelial regrowth was noted 14–28 days after insertion of sirolimus-eluting stents, thus suggesting a low risk of late thrombosis. The sirolimus-eluting stent was also not associated with vessel wall thinning or necrosis, positive remodelling, or aneurysm.
Pharmacokinetic Properties
A minimal quantity of sirolimus is released from the sirolimus-eluting stent into the bloodstream. After sirolimus-eluting stent insertion in 19 patients with coronary artery disease, the mean peak blood sirolimus concentration (Cmax) was 0.80 ng/mL attained 3.59 hours after stent implantation. This Cmax value was approximately 20–400 times lower than that reported in ascending single-oral dose studies of sirolimus in renal transplant recipients. In vivo animal studies generally revealed low tissue sirolimus levels (0.5–2 ng/mg) in the stent borders during the 30-day period after sirolimus-eluting stent insertion. Higher peak tissue levels were noted when high-content sirolimus-eluting stents were implanted, although sirolimus levels in peri-stent arterial and myocardial tissue remained low (<1 ng/mg) throughout the 30-day study period. In other animal studies, the residual in-stent sirolimus content 28 days after stent implantation was approximately 30% of the original amount.
The apparent terminal-phase volume of distribution for sirolimus after sirolimus-eluting stent insertion in patients with coronary artery disease was high (407L). Sirolimus is extensively metabolised and is principally cleared in the feces but this is not likely to be important with the sirolimus-eluting stent. Although the potential for clinically significant drug interactions cannot be totally excluded, the low quantities of sirolimus released from the sirolimus-eluting stent into the bloodstream suggest that such interactions are unlikely.
Therapeutic Use
In single de novo native coronary artery lesions, the sirolimus-eluting stent (140 μg/cm2 of sirolimus) was associated with only minimal neointimal hyperplasia (NIH) during follow-up periods of up to 2 years. In an uncontrolled study, no patients had in-stent restenosis, as measured by quantitative coronary angiography (n = 28), approximately 2 years after implantation of a fast- or slow-release sirolimus-eluting stent in lesions ≤18mm in length in medium size vessels, and event-free survival in 45 patients was 92%.
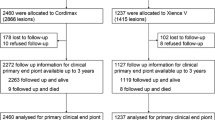
In four well-designed comparative trials, the incidence of major adverse cardiac events (MACE) was significantly lower in patients who received a sirolimus-eluting stent than in patients who received an uncoated stent. In the RAndomized study with the sirolimus-coated Bx VELocity™ stent (RAVEL) [n = 238], the incidence of MACE at 1 year was 5.8% in the sirolimus-stent group (n = 120) compared with 28.8% in the uncoated-stent group (n = 118) [p < 0.001]. The US (n = 1058), European (n = 352) and Canadian (n = 100) studies of the SIRolImUS-eluting stent in de novo coronary lesions (SIRIUS) included more complex lesions and patients with more cardiac risk factors than the RAVEL trial. At 9-month follow-up in the SIRIUS trials, the incidence of all MACE ranged from 4–8% in the sirolimus-eluting stent group compared with 18–23% in the uncoated-stent group (p < 0.05). In all studies, the difference in MACE was due to the higher need for repeat percutaneous revascularization of the target vessel in the group who received uncoated stents than in those who received sirolimus-eluting stents.
At 6-month angiographic follow-up (n = 211) in the RAVEL trial, mean late luminal loss (LLL) in the stented segment and at the proximal and distal edges, in-stent stenosis, NIH, and volume obstruction were all significantly lower in the sirolimus-stent group than in the uncoated-stent group (p < 0.05 for all comparisons). The percentage of patients with ≥50% in-stent restenosis at 6 months was 0% in the sirolimus-stent group compared with 27% in the uncoated-stent group (p < 0.001). At 8-month angiographic follow-up in the SIRIUS studies, in-stent and in-segment LLL and restenosis were all significantly different between treatment groups in favor of the sirolimus-stent group (p < 0.001 for all comparisons).
In in-stent restenosis, the efficacy of sirolimus-eluting stents was similar to that achieved in de novo lesions, and in a preliminary report, efficacy with the sirolimus-eluting stent appeared better than that achieved with brachytherapy.
Despite the higher acquisition cost of the sirolimus-eluting stent, cost-effectiveness analyses have shown a cost advantage for the sirolimus stent compared with an uncoated stent at 12-month follow-up, due to a reduced requirement for repeat target vessel revascularization.
Tolerability
In clinical trials to date, including up to 3 years’ follow-up, the sirolimus-eluting stent was well tolerated. At 6-month angiographic follow-up in the RAVEL study (n = 238), there was no evidence of in-stent thrombosis, edge effect, aneurysm formation, or persistent dissection in patients who received sirolimus-eluting stents. In the three SIRIUS studies, which included >1500 patients, the incidence of subacute and late thrombosis was no different between the sirolimus-eluting stent group and the uncoated-stent group. In-stent thrombosis was observed at 6-month follow-up in 3 of 85 patients who received one or two sirolimus-eluting stents in the Bifurcation Trial, but was not evident in any patients (n = 25) at 12-month follow-up who received one or two sirolimus-eluting stents for the treatment of in-stent restenosis. In both the RAVEL and US SIRIUS studies, patients with diabetes appear to have tolerated the sirolimus-eluting stent as well as the total patient populations.
To date, incomplete apposition is the only potentially adverse event that has occurred more frequently in the sirolimus-stent group than in the uncoated-stent group; however, these events were not associated with any clinical consequence.







Similar content being viewed by others
Notes
Use of tradename is for product identification purposes only and does not imply endorsement.
References
Curfman GD. Sirolimus-eluting coronary stents. N Engl J Med 2002 Jun 6; 346(23): 1770–1
El-Omar MM, Dangas G, Iakovou I., et al. Update on in-stent restenosis. Curr Interv Cardiol Rep 2001; 3: 296–305
Cordis Corporation. Final results of large-scale U.S. study confirm positive performance of Cordis CYPHER sirolimus-eluting stent. Media Rel 2002 Sep 24
Cordis Corporation. Instructions for Use: CYPHER sirolimus-eluting coronary stent on RAPTOR over-the-wire delivery system and CYPHER sirolimus-eluting coronary stent on RAPTORAIL rapid exchange delivery system [online]. Available from URL: www.cypherusa.com [Accessed 2003 May 19]
Duda SH, Pusich B, Richter G, et al. Sirolimus-eluting stents for the treatment of obstructive superficial femoral artery disease: six-month results. Circulation 2002 Sep 17; 106(12): 1505–9
Moses JW, Kipshidze N, Leon MB. Perspectives of drug eluting stents: the next revolution. Am J Cardiovasc Drug 2002; 2(3): 163–72
Sehgal SN, Camardo JS, Scarola JA, et al. Rapamycin (sirolimus, rapamune). Curr Opin Nephrol Hypertens 1995 Nov; 4: 482–7
Sehgal SN. Rapamune (sirolimus, rapamycin): an overview and mechanism of action. Ther Drug Monit 1995 Dec; 17: 660–5
Molnar-Kimber KL. Mechanism of action of rapamycin (sirolimus, Rapamune). Transplant Proc 1996 Apr; 28: 964–9
Brazelton TR, Morris RE. Molecular mechanisms of action of new xenobiotic immunosuppressive drugs: tacrolimus (FK506), sirolimus (rapamycin), mycophenolate mofetil and leflunomide. Curr Opin Immunol 1996 Oct; 8: 710–20
Sehgal SN. Rapamune (Rm) (RAPA, rapamycin, sirolimus): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem 1998 Jul; 31: 335–40
Ingle GR, Sievers TM, Holt CD. Sirolimus: continuing the evolution of transplant immunosuppression. Ann Pharmacother 2000 Sep; 34(9): 1044–55
Napoli KL, Taylor PJ. From beach to bedside: history of the development of sirolimus. Ther Drug Monit 2001 Oct; 23(5): 559–86
Poon M, Badimon JJ, Fuster V. Overcoming restenosis with sirolimus: from alphabet soup to clinical reality. Lancet 2002 Feb 16; 359(9306): 619–22
Anonymous. Sirolimus: AY 22989, NSC 226080, NSC 606698, rapamycin, Rapamune®. Drugs R & D 1999; 1 (1): 100-7
Fattori R, Piva T. Drug-eluting stents in vascular intervention. Lancet 2003; 361: 247–9
Klugherz BD, Llanos G, Lieuallen W, et al. Twenty-eight-day efficacy and phamacokinetics of the sirolimus-eluting stent. Coron Artery Dis 2002 May; 13(3): 183–8
Toutouzas K, Di Mario C, Falotico R, et al. Sirolimus-eluting stents: a review of experimental and clinical findings. Z Kardiol 2002; 91 Suppl. 3: III/49–57
Molnar-Kimber KL, Rhoad A, Warner L, et al. Comparison of effects of sirolimus on cytokine dependent and cytokine independent proliferation. Inflamm Res 1995 Aug; 44 Suppl. 2: S189–190
Suzuki T, Kopia G, Hayashi S, et al. Stent-based delivery of sirolimus reduces neointimal formation in a porcine coronary model. Circulation 2001 Sep 4; 104(10): 1188–93
Carter AJ, Tio F, Bailey L, et al. A sirolimus eluting smart stent reduces inflammation and neointimal formation in a canine model. Circulation 2001 Oct 23; 104: 764
Matter CM, Kurz DJ, Wnendt S, et al. Tacrolimus (FK506), but not sirolimus (rapamycin) targets human vascular smooth muscle cells, but spares endothelial cells: implications for drug-eluting stents. Circulation 2002 Nov 5; 106 Suppl.: II356–357
Poon M, Marx SO, Gallo R, et al. Rapamycin inhibits vascular smooth muscle cell migration. J Clin Invest 1996; 15(98): 2277–83
Lohrmann JD, Franke GN, Kollum MK, et al. Paclitaxel but not rapamycin induces plasminogen activator inhibitor type-1 expression in human vascular smooth muscle cells [abstract no. 2224]. Circulation 2002 Nov 5; 106 Suppl.: II–447
Aggarwal M, Tsao PS, Kopia G, et al. Stent based delivery of sirolimus is associated with increased levels of p27Kip1 without long-term suppression of neointimal hyperplasia in the porcine model. Circulation 2002 Nov 5; 106 Suppl.: II–357
Kopia GA, Snead D, Faloticol R, et al. Pharmacokinetics and pharmacodynamics of sirolimus-eluting stents [abstract no. 290]. Am J Cardiol 2002; 90 Suppl. 6A: 115H
Kopia GA, Llanos G, Falotico R, et al. Sirolimus-eluting stents: antirestenosis efficacy versus local and systemic pharmacokinetics in a porcine coronary model [abstract no. 295]. Am J Cardiol 2002; 90 Suppl. 6A: 117H
Guyon P, Teiger E, Chevalier B, et al. Rapamycin coated stent effectiveness in high arterial injury damage: a pig model experimentation [abstract no. 2232]. Circulation 2002 Nov 5; 106 Suppl.: II–449
Lauer MA, Kovalcsik RM, DeVries JJ, et al. Sirolimus-eluting self-expanding nitinol stents dramatically reduce restenosis in a canine model of iliac stenting [abstract no. 3523]. Circulation 2002; 106 (19 Suppl.): II–714
Kopia GA, Falotico R, Gallagher L, et al. Sirolimus-eluting stents: regrowth of endothelial cells after stent implantation [abstract no. 284]. Am J Cardiol 2002; 90 Suppl. 6A: 113H
Gijsen FJH, Oortman RM, Wentzel JJ, et al. Blood flow pattern predicts neointima thickness distribution in sirolimus coated stents [abstract no. 2884]. Circulation 2002; 106 Suppl.: II–584
Mahalati K, Kahan BD. Clinical pharmacokinetics of sirolimus. Clin Pharmacokinet 2001; 40(8): 573–85
Gallant-Haidner HL, Trepanier DJ, Freitag DG, et al. Pharmacokinetics and metabolism of sirolimus. Ther Drug Monit 2000 Feb; 22(1): 31–5
Vetrovec GW, Rizik D, Goudreau E, et al. The Sirolimus PK trial: a pharmacologic study of the sirolimus-eluting BX velocity stent in patients with de novo coronary lesions [abstract no. 1775]. Circulation 2002 Nov 5; 106 Suppl.: II–355
Morice M-C, Serrays PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med 2002 Jun 6; 346(23): 1773–80
Moses JW, Leon MB, Popma JJ, et al. A multicenter randomized clinical study of the sirolimus-eluting stent in native coronary lesions: clinical outcomes [abstract no. 1951]. Circulation 2002 Nov 5; 106 Suppl.: II392–393. Plus oral presentation at American Heart Association Meeting, 2002 Nov 17–20; Chicago (IL)
Schofer J, Schluter M. E-SIRIUS: 8-month efficacy results. Oral presentation at the 52nd Annual Scientific Session of the American College of Cardiology; 2003 Mar 30–Apr 2; Chicago (IL)
Schampaert E, Cohen EA, Reeves F, et al. Results from the Canadian multicenter, randomized, double-blind study of the sirolimus-eluting stent in the treatment of patients with de novo coronary artery lesions. Oral presentation at the 52nd Annual Scientific Session of the American College of Cardiology; 2003 Mar 30–Apr 2; Chicago (IL)
Degertekin M, Serruys PW, Foley DP, et al. Persistent inhibition of neointimal hyperplasia after sirolimus-eluting stent implantation: long-term (up to 2 years) clinical, angiographic, and intravascular ultrasound follow-up. Circulation 2002 Sep 24; 106(13): 1610–3
Sousa JE, Costa MA, Sousa AG, et al. Two-year angiographic and intravascular ultrasound follow-up after implantation of sirolimus-eluting stents in human coronary arteries. Circulation 2003 Jan 28; 107(3): 381–3
Sousa JE, Costa MA, Abizaid AC, et al. Sustained suppression of neointimal proliferation by sirolimus-eluting stents: one-year angiographic and intravascular ultrasound follow-up. Circulation 2001 Oct 23; 104(17): 2007–11
Sousa JE, Abizaid A, Abizaid A, et al. Late (three-year) follow-up from the first-in-man (FIM) experience after implantation of sirolimus-eluting stents [abstract no. 1958]. Circulation 2002 Nov 5; 106 Suppl.: II–394. Plus oral presentation at American Heart Association Meeting, 2002 Nov 17–20; Chicago (IL)
Regar E, Serruys PW, Bode C, et al. Angiographic findings of the multicenter Randomized Study With the Sirolimus-Eluting Bx Velocity Balloon-Expandable Stent (RAVEL): sirolimus-eluting stents inhibit restenosis irrespective of the vessel size. Circulation 2002 Oct 8; 106(15): 1949–56
Sousa JM. 3-Year follow-up of the first-in-man sirolimus patients: are there any late concerns? Oral presentation at the 52nd Annual Scientific Session of the American College of Cardiology; 2003 Mar 30–Apr 2; Chicago (IL)
Morice M-C, Serruys PW, Constantini C, et al. Two-year follow-up of the RAVEL study: a randomized study with the sirolimus-eluting Bx VELOCITY stent in the treatment of patients with de-novo native coronary artery lesions [abstract no. 805-1]. Am J Coll Cardiol 2003 Mar 31; 41 (6 Suppl. A): 32A. Plus oral presentation at the 52nd Annual Scientific Session of the American College of Cardiology, 2003 Mar 30–Apr 2; Chicago (IL)
Moses JW, O’shaughnessy C, Caputo R, et al. The U.S. multicenter, randomized, double-blind study of the sirolimus-eluting stent in coronary lesions: safety outcomes at 9 months [abstract no. 1453]. Eur Heart J 2002 Sep; 23 Suppl.: 264
Holmes Jr DR, Leon MB, Moses JW, et al. One-year follow-up of the SIRIUS study: a randomized study with the sirolimus-eluting Bx VELOCITY in the treatment of patients with de-novo native coronary artery lesions [abstract no. 805-3]. J Am Coll Cardiol 2003; 41 (6 Suppl. A): 32A–3A
Moses JW. Insights from the SIRIUS trial. Oral presentation at the 52nd Annual Scientific Session of the American College of Cardiology; 2003 Mar 30–Apr 2; Chicago (IL)
Ako J, Morino Y, Honda Y, et al. Effects of sirolimus-eluting stents in diabetic patients: volumetric intravascular ultrasound analysis from the SIRIUS trial [abstract no. 1198-180]. J Am Coll Cardiol 2003; 41 (6 Suppl. A): 73A
Leon MB, Holmes DR, Simonton C, et al. The impact of sirolimus-eluting stents in diabetics: results from the SIRIUS trial [abstract no. 839FO-6]. J Am Coll Cardiol 2002; 41 (6 Suppl. A): 54A
Popma JJ, Cox NR, Moses JW, et al. Effect of vessel size on angiographic restenosis in a randomized comparison of bare metal and sirolimus-eluting Bx Velocity stents: a SIRIUS trial substudy [abstract no. 2567]. Circulation 2002 Nov 5; 106 Suppl.: II–520
Mauri L, Popma JJ, Fitzgerald PJ, et al. Predictors of clinical and angiographic restenosis in the sirolimus-eluting Bx VELOCITY stent trial [abstract no. 813-3]. J Am Coll Cardiol 2003 Mar; 41 (6 Suppl. A): 35A
Leon MB, Popma JJ, Yakubov S, et al. The effects of sirolimus-eluting stents on in-stent and peri-stent restenosis: an angiographic subanalysis from the SIRIUS trial [abstract no. 1030-186]. J Am Coll Cardiol 2003 Mar; 3 (6 Suppl. A): 14A
Ako J, Morino Y, Honda Y, et al. Serial stent edge analysis following sirolimus eluting stent implantation: interim IVUS results from the SIRIUS trial [abstract no. 2564]. Circulation 2002 Nov 5; 106 Suppl.: II–519
Morino Y, Ako J, Honda Y, et al. Overlapping sirolimus-eluting stents: intravascular ultrasound analysis from the SIRIUS trial [abstract no. 877-5]. J Am Coll Cardiol 2003 Mar; 41 (6 Suppl. A): 81A
Weisz G, Moses JW, Popma JJ, et al. Do overlapping multiple sirolimus-eluting stents impact angiographic and clinical outcomes? Insights from the SIRIUS trial [abstract no. 805-5]. J Am Coll Cardiol 2003 Mar; 41 (6 Suppl. A): 33A
Abizaid AA, Munoz JS, Seixas AC, et al. Is there any arterial toxic effect after overlapping sirolimus-eluting stents? [abstract no. 1030-185] J Am Coll Cardiol 2003 Mar; 41 (6 Suppl. A): 13A–4A
Shah J, Bakhai A, Wong A, et al. Evaluating the impact of geographical miss and lesion miss on late lumen loss in the SIRIUS trial. Heart, 89 Suppl. 1: A53. Plus oral presentation at British Cardiac Society Meeting; 2003 Apr 28–May 01; Glasgow
Foley DP, Melkert R, Serruys PW. Influence of coronary vessel size on renarrowing process and late angiographic outcome after successful balloon angioplasty. Circulation 1994; 90: 1239–51
Tanabe K, Serruys PW, Degertekin M, et al. Fate of side branches after coronary arterial sirolimus-eluting stent implantation. Am J Cardiol 2002 Nov 1; 90(9): 937–41
Schofer J, Breithardt G, Kuntz RE, et al. A European multi-centre, randomised, double-blind study of the sirolimus-eluting stent in patients with de novo coronary artery lesions [abstract no. 1454]. Eur Heart J 2002 Sep; 23 Suppl.: 265. Plus oral presentation at the 24th Congress of the European Society of Cardiology, 2002 Aug 31–Sep 4; Berlin
Colombo A, Leon MB, Morice MC, et al. The BIFURCATION study: an evaluation of the CYPHER™ sirolimus-eluting stent in the treatment of patients with bifurcation lesions [abstract no. 2390]. Circulation 2002 Nov 5; 106 Suppl.: II483–484. Plus oral presentation at the American Heart Association Meeting, 2002 Nov 17–20; Chicago (IL)
Colombo A, Louvard Y, Raghu C, et al. Sirolimus-eluting stents in bifurcation lesions: six month angiographic results according to the implantation technique [abstract no. 839FO-2]. J Am Coll Cardiol 2003 Mar; 41 (6 Suppl. A): 53A
Degertekin M, Regar E, Tanabe K, et al. Sirolimus-eluting stent for treatment of complex in-stent restenosis: the first clinical experience. J Am Coll Cardiol 2003 Jan 15; 41(2): 184–9
Sousa JE, Costa MA, Abizaid A, et al. Sirolimus-eluting stent for the treatment of in-stent restenosis: a quantitative coronary angiography and three-dimensional intravascular ultrasound study. Circulation 2003 Jan 7; 107(1): 24–7
Degertekin M, Regar E, Tanabe K, et al. Sirolimus eluting stents prevent neointimal growth regardless of lesions type (de-novo, in-stent restenosis): a 3D IVUS analysis [abstract no. 3206]. J Am Coll Cardiol 2002 May 1; 39 Suppl. B: 21 OB
Leon MB, Moses JW, Weisz G, et al. The frequency and consequences of angiographic aneurysms after sirolimus-eluting stents: results from SIRIUS [abstract no. 1030-183]. J Am Coll Cardiol 2003 Mar; 41 (6 Suppl. A): 13A
Serruys PW, Degertekin M, Tanabe K, et al. Intravascular ultrasound findings in the multicenter, randomized, double-blind RAVEL (RAndomized study with the sirolimus-eluting VElocity balloon-expandable stent in the treatment of patients with de novo native coronary artery lesions) trial. Circulation 2002 Aug 13; 106(7): 798–803
Ako J, Morino Y, Honda Y, et al. Late incomplete stent apposition following sirolimus-eluting stent: serial quantitative intravascular ultrasound analysis from the SIRIUS trial [abstract no. 805-4]. J Am Coll Cardiol 2003 Mar; 41 (6 Suppl. A): 33A
Johnson & Johnson. CYPHER sirolimus-eluting stent. Prescribing Information [online]. Available from URL: http//www.jnjgateway.com [Accessed 2003 Mar 30]. 2002
van Hout BA, Lindeboom WK, Morice MC, et al. Cost-effectiveness of the sirolimus eluting Bx-VELOCITY stent: 1-year results [abstract no. P3574]. Eur Heart J 2002; 23 Suppl.: 691
Cohen DJ, Bakhai A, Shi C, et al. Cost-effectiveness of sirolimus-eluting stent for treatment of complex coronary stenoses: results from the randomized SIRIUS trial [abstract no. 805-2]. J Am Coll Cardiol. 2003; 41 Suppl. A: 32A. Plus oral presentation at the 52nd Annual Scientific Session of the American College of Cardiology; 2003 Mar 30–Apr 2; Chicago (IL)
Lamotte M, Annemans L, De Jong P. Drug-eluting stents in coronary artery disease: assessment of outcomes and cost effectiveness [abstract no. 810]. Eur Heart J 2002 Aug–2002 30; 23 Suppl.: 137
Marchetti M, Tarricone R, Lamotte M, et al. Cost-effectiveness and budget impact of the sirolimus-eluting stent in the stent era [abstract no. HS1]. Value Health 2002 Nov–2002 31;5: 457
Serruys PW, Unger F, Sousa JE, et al. Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. N Engl J Med 2001; 344: 1117–24
Fischman DL, Leon MB, Baim DS, et al. A randomized comparison of coronary stent placement and balloon angioplasty in the treatment of coronary artery disease. N Engl J Med 1994; 331: 496–501
Serruys PW, de Jaegere P, Kiemeneij F, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. N Engl J Med 1994; 331(8): 489–95
Beyar R, Roguin A. The sirolimus coated stent: will the Achilles heel of interventional cardiology finally be cured? Eur Heart J 2001 Nov; 22(22): 2054–7
Degertekin M, Regar E, Tanabe K, et al. Sirolimus eluting stent in the treatment of atherosclerosis coronary artery disease. Minerva Cardioangiol 2002 Oct; 50(5): 405–18
Leon MB, Teirstein PS, Moses JW, et al. Localized intracoronary gamma-radiation therapy to inhibit the recurrence of restenosis after stenting. N Engl J Med 2001; 344(4): 250–6
Feres F, Munoz JS, Abizaid AA, et al. Sirolimus-eluting stent or intracoronary brachytherapy to treat in-stent restenosis [abstract no. 879-6]. J Am Coll Cardiol 2003 Mar; 41 (6 Suppl. A): 83A
Morino Y, Ako J, Sonoda S, et al. Neointimal distribution within sirolimus-eluting stents: a 3-D IVUS interim analysis from the SIRIUS trial [abstract no. 1946]. Circulation 2002 Nov; 106 (19 Suppl.): II–391
Costa MA, Moses JW, Leon MB, et al. Sirolimus-eluting stent for the treatment of bypass graft disease: the initial U.S. experience [abstract no. 1030-183]. J Am Coll Cardiol 2003; 41 (6 Suppl. A): 13A
Teirstein PS, Kao J, Bass TA, et al. Use of the sirolimus-eluting Bx VELOCITY stent for failed brachytherapy in recurrent in-stent restenosis: results from the SECURE registry [abstract no. 1128-178]. J Am Coll Cardiol 2003; 41 (6 Suppl. A): 48A
Saia F, Lemos PA, Degertekin M, et al. Sirolimus-eluting stents for treatment of in-stent restenosis in the real world: preliminary results from the rapamycineluting stent evaluated at Rotterdam Cardiology Hospital (RESEARCH) registry [abstract no. 839F0-4]. J Am Coll Cardiol 2003 Mar; 41 (6 Suppl. A): 53A
Tanabe K, Lemos PA, Lee C, et al. The impact of sirolimus-eluting stents on the outcome of patients with bifurcation lesions [abstract no. 1030-180]. J Am Coll Cardiol 2003; 41 (6 Suppl. A): 12A
Lee CH, Lemos PA, van Domburg RT, et al. Safety and efficacy of sirolimus-eluting stent (cypher) in acute myocardial infarction: a substudy of the rapamycin-eluting stent evaluation at Rotterdam Cardiology Hospital (RESEARCH) study [abstract no. 1052-186]. J Am Coll Cardiol 2003; 41 (6 Suppl. A): 21A
Regar E, Lemos PA, Lee C-H, et al. Subacute stent thrombosis after sirolimus-eluting stent implantation in daily practice: results from the rapamycin elutingstent evaluated at Rotterdam Hospital (RESEARCH) registry [abstract no. 1053-202]. J Am Coll Cardiol 2003; 41 (6 Suppl. A): 23A
Bertrand ME, Simoons ML, Fox AA, et al. Management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Task Force on the Management of Acute Coronary Syndromes of the European Society of Cardiology. Eur Heart J 2002; 23: 1809–40
Medtronic. Medtronic announces start of endeavor drug-eluting stent trial [media release]. 2003
Grube E, Buellesfeld L, Mueller R, et al. First human experience using a new Everolimus stent coating: procedural and six-month follow-up results of the FUTURE trial [abstract no. 1006-183]. J Am Coll Cardiol 2003; 41 (6 Suppl. A): 6A
Grube E, Silber S, Hauptmann KE, et al. TAXUS I: six- and twelve-month results from a randomized, double-blind trial on a slow-release paclitaxel-eluting stent for de novo coronary lesions. Circulation 2003 Jan; 107: 38–42
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by
A. Bakhai, Interventional Cardiology, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA; A. Colombo, Cardiac Catheterization Laboratory, EMO Centro Cuore Columbus, Milano, Italy; J.-M. Juliard, Interventional Cardiology Department, Bichat Hospital, Paris, France; T. Lefévre, Institut Hospitalier Jacques Cartier, Massy, France; A.M. Lincoff, Department of Cardiovascular Medicine, The Cleveland Clinic Foundation, Cleveland, Ohio, USA; J.W. Moses, Lenox Hill Heart and Vascular Institute and Cardiovascular Research Foundation, New York, New York, USA; R. Wilensky, Experimental Interventional Cardiology, University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Data Selection
Sources: Medical literature published in any language since 1980 on sirolimus, identified using Medline and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: Medline search terms were ‘sirolimus’ and (‘eluting’ or ‘stent’). EMBASE search terms were ‘sirolimus’ and (‘eluting’ or ‘stent’). AdisBase search terms were ‘sirolimus’ and (‘eluting’ or ‘stent’). Searches were last updated 12 May 2003.
Selection: Studies in patients with coronary artery disease who received sirolimus-eluting stents. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Drug-eluting stents, pharmacodynamics, pharmacokinetics, sirolimus-eluting stents, therapeutic use, vascular intervention.
Rights and permissions
About this article
Cite this article
McKeage, K., Murdoch, D. & Goa, K.L. The Sirolimus-Eluting Stent. Am J Cardiovasc Drugs 3, 211–230 (2003). https://doi.org/10.2165/00129784-200303030-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00129784-200303030-00007