Summary
Synopsis
Interferon- α- n1 (lymphoblastoid interferon- α) is a nonrecombinant ‘natural’ interferon derived from lymphoblastoid cells exposed to Sendai virus. In common with endogenous and recombinant interferon- α molecules, interferon- α- n1 has antiviral, immunomodulatory and antiproliferative properties.
Interferon- α- n1 shows some efficacy in immunocompetent adults with well- compensated chronic viral hepatitis B. Rates of complete virological response (defined as an absence of detectable hepatitis B virus- DNA in the serum) ranged from 5 to 79% of adults who received various dosage regimens of interferon- α- n1 in monotherapy trials. Clearance of hepatitis B ‘e’ antigen was reported in 5 to 70% of patients treated with the drug. Spontaneous virological responses occurred in 0 to 48% of untreated patients.
The clinical efficacy of interferon- α- n1 in patients with chronic hepatitis B is not improved by concomitantly administered deflazacort, zidovudine or levamisole, but may be increased by a course of corticosteroid pretreatment in some patients.
Interferon- α- n1 also shows therapeutic benefit in adults with chronic hepatitis C. Complete biochemical responses (defined as normalisation of serum ALT levels) were achieved in 27 to 60% of adult patients treated with the drug, whereas spontaneous normalisation of serum ALT levels occurred in up to 11% of untreated patients. Responses to interferon- α- nl were temporary in 27 to 78% of treatment responders but were sustained in 6 to 40% of patients. Emerging data delineating baseline factors predictive of a positive response to interferon- a- nl treatment may aid in the selection of patients with hepatitis B or C most likely to benefit from treatment with this drug.
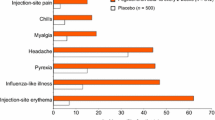
Most patients receiving interferon- a- nl experience a transient ‘influenza- like ’ syndrome during the first week of treatment. The syndrome, which is dose related and alleviated by paracetamol (acetaminophen), is characterised by fever, chills, and arthralgia. Dose- limiting adverse effects occurring during longer term interferon- a- n1 therapy include fatigue, myalgia, headache, depression, pruritus and seizures. Neutropenia and thrombocytopenia may also occur during interferon- α- nl treatment. Autoimmune thyroid disease may develop in up to 9% of patients treated with interferon- α- nl for ≥ 6 months.
At present, interferon- α- n1 and the recombinant forms of interferon- α are the only drugs available for the treatment of adults with well-compensated hepatitis B or C. Interferon- α- nl produces moderate response rates in adults with well-compensated chronic hepatitis B or C. Thus, it is positioned alongside recombinant interferon- a products as a useful first-line treatment option for patients with chronic hepatitis B or C.
Pharmacological Properties
Interferon-α-n1 is a nonrecombinant mixture of ‘natural» interferon-α subtypes. It is produced in vitro from human lymphoblastoid cells exposed to Sendai virus and thus is also referred to as lymphoblastoid interferon-α.
The drug produces antiviral effects by inducing the production of ≥20 effector proteins (notably 2′,5′-oligoadenylate synthetase and protein kinase), which block viral replication in the host cell. Interferon-α-n1 also inhibits the intracellular accumulation of replicative hepatitis B virus (HBV)-RNA.
Increased expression of cell membrane components, macrophage activation and modulation of natural killer and cytotoxic T cell activity are among the numerous documented immunomodulatory properties of interferon-a-nl. Interferon-α-n1 also has antiproliferative activity in vitro and in vivo. Evidence for an antitumour effect of interferon-α-nl in murine xenograft models is conflicting.
Interferon-α-n1 has a lower propensity than either interferon-α-2a or interferon -α-2b to stimulate the production of neutralising antibodies in patients with chronic hepatitis B or C.
Inhibition of hepatic regenerative activity was more marked in rats exposed to interferon-α-2a than in animals exposed to either interferon-α-n1 or interferon-α- 2b and was seen only with higher dose equivalents of interferon-α-n1 or interferon-α-2b than those used clinically.
The pharmacokinetics of interferon-α-n1 are similar after subcutaneous and intramuscular administration. Maximum serum interferon-α-n1 concentrations of 200 to 300 IU/ml occur after multiple doses of up to 15MU administered intramuscularly or subcutaneously 3 times weekly. Maximum serum drug concentrations are usually observed 4 to 8 hours after subcutaneous or intramuscular administration. Interferon-α-n1 is not usually detectable in the urine, although it may be present in the urine of patients with severe renal disease.
Therapeutic Efficacy
Chronic Hepatitis B. Complete virological responses (defined as an absence of detectable levels of HBV-DNA in the serum) occurred in 5 to 79% of recipients of interferon-α-n1 either at treatment completion, or up to 12 months after discontinuation of treatment. Complete virological responses were also evident in 0 to 48% of patients receiving placebo or no treatment. The drug was generally administered in maintenance dosages of 2.5 to 5 MU/m2 3 times weekly for up to 6 months. Serum hepatitis B ‘e’ antigen (HBeAg) was cleared in 5 to 70% of patients with chronic hepatitis B receiving interferon-α-n1, compared with 0 to 39% of placebo recipients or untreated controls, suggesting spontaneous responses in some untreated patients.
The efficacy of interferon-α-n1 in patients with chronic hepatitis B is not improved by concomitantly administered zidovudine, levamisole or deflazacort. However, results of one double-blind trial indicated that a 1-month course of prednisolone treatment before initiation of interferon-α-n1 therapy significantly improved response rates, compared with interferon-α-n1 alone.
Chronic Hepatitis C. Results of numerous trials conducted in adults with chronic hepatitis C showed that interferon-α-n1 produced complete responses (defined as normalisation of serum ALT levels by the end of the treatment period) in 27 to 60% of patients. Spontaneous normalisation of serum ALT levels occurred in 0 to 11% of untreated patients. Rates of sustained response ranged from 6 to 40%; a proportion of treatment responders (27 to 78%) relapsed after treatment discontinuation (most within 2 to 3 months of finishing treatment).
Preliminary results from a large clinical trial involving more than 1000 patients indicate that interferon-α-n1 treatment may produce higher sustained response rates than interferon-α-2b. However, sustained response rates were similar for patients receiving interferon-α-n1 and interferon-α-2a in another trial.
The onset of hepatitis C ‘breakthrough’ (defined as an increase in serum ALT levels after a period of interferon-α-induced normalisation) is attributed to interferon-α-specific cell receptor down-regulation, the appearance of anti-interferon-α neutralising antibodies and the emergence of viral mutants resistant to the drug.
Interferon-α-n1 may restore therapeutic response in patients who do not respond to treatment with interferon-α-2a or -2b because of the development of neutralising antibodies.
Evidence from several clinical trials indicates that interferon-α-n1 treatment may contribute towards preventing the development of hepatocellular carcinoma in patients with chronic viral hepatitis.
Predictors of Response
Low baseline serum HBV-DNA levels, high serum ALT levels and active hepatic disease with fibrosis appear to be the best predictors of a positive response to interferon-α treatment in patients with chronic hepatitis B. Response predictors in patients with hepatitis C include low baseline viral RNA levels, minimal fibrosis, the absence of cirrhosis and the absence of hepatitis C virus genotype 1b.
Tolerability
The tolerability profile of interferon-α-n1 is typical of the interferons-α as a class. Adverse effects occur frequently and are associated with the dosage and duration of interferon-α-n1 treatment. Patients with cirrhosis are more likely to experience adverse effects than noncirrhotic patients.
A transient dose-related ‘influenza-like’ illness, manifest as fever, myalgia, headache, chills and arthralgia, is experienced by most patients during the first week of treatment with the drug. These symptoms are usually ameliorated by paracetamol (acetaminophen). Adverse effects of interferon-α-n1 occurring during longer term treatment (≥2 weeks) include fatigue, myalgia, headache, depression, anorexia, pruritus, seizures and hair loss. Haematological suppression, such as neutropenia and thrombocytopenia, occurs in some recipients of interferon-α-n1. Although tolerance to some adverse effects may develop, dosage reduction is required for some patients.
Some autoimmune diseases are triggered during long term treatment with interferon-α-n1. Autoimmune thyroid disease may develop in up to 9% of patients during ≥6 months’ interferon-α-n1 treatment. After treatment discontinuation, thyroid dysfunction persists in some patients but resolves in others.
Dosage and Administration
Interferon-α-n1 is administered either subcutaneously or intramuscularly; rotation of the site of injection is advised.
In clinical trials conducted in adult patients with well-compensated chronic hepatitis B, interferon-α-n1 was generally administered in induction dosages of 1.25 to 6 MU/m2 /day (for 1 to 4 weeks) followed by maintenance dosages of 2.5 to 5 MU/m 3 times weekly for up to 6 months. The manufacturer recommends a dosage of 10 to 15MU (to a maximum of 7.5 MU/m ) 3 times weekly for 12 weeks. Alternatively, a lower dosage of 5 to 10MU 3 times weekly may be given for up to 6 months.
The dosage of interferon-α (subtype not specified) recommended by the US National Institutes of Health for the initial treatment of patients with chronic hepatitis C is 3MU 3 times daily for 12 months. The manufacturer of the drug currently recommends a dosage of 3 to 5MU 3 times weekly for up to 48 weeks.
Similar content being viewed by others
References
Cirelli R, Tyring SK. Major therapeutic uses of interferons. Clin Immunother 1995; 3(1): 27–87
Dianzani F. Biological basis for the clinical use of interferon. Gut 1993: S74-6
Farrell GC. Therapy of hepatitis C: Interferon alfa-n1 trials. Hepatology 1997; 26Suppl. 1: 96S–100S
Dorr RT. Interferon-α in malignant and viral diseases: a review. Drugs 1993; 45(2): 177–211
Wellcome UK. Wellferon. Interferon alfa-n1. Prescribing information. ABPI Compendium of Data Sheets and Summaries of Product Characteristics 1996–7. Datapharm Publications Limited. London, UK
Davis MG, Jansen RW. Inhibition of hepatitis B virus in tissue culture by alpha interferon. Antimicrob Agents Chemother 1994 Dec; 38: 2921–4
Staeheli P. Interferon-induced proteins and the antiviral state. Adv Virus Res 1990; 38: 147–200
Nouri-Aria KT, Alexander GJM, Magrin S, et al. Differential effect of α-interferons on CD4- and CD8-positive lymphocytes in chronic hepatitis B virus carriers. J Hepatol 1988; 7(1): 1–6
Lucas M, Serejo F, Valente A, et al. Changes in peripheral blood lymphocyte subsets in chronic hepatitis C: differences between α-2b and α-n1 interferon [abstract]. Hepatology 1993 Oct; 18 (Pt 2): 241A
Castilla A, Camps Bansell-J, Civeira M-P, et al. Lymphoblastoid α-interferon for chronic hepatitis C: a randomized controlled study. Am J Gastroenterol 1993; 88(2): 233–9
Dunk AA, Ikeda T, Pignatelli M, et al. Human lymphoblastoid interferon. In vitro and in vivo studies in hepatocellular carcinoma. J Hepatol 1986; 2(3): 419–29
Dunk AA, Novick D, Thomas HC. Natural killer cell activity in hepatocellular carcinoma. In vitro and in vivo responses to interferon. Scand J Gastroenterol 1987; 22(10): 1245–50
Andreone P, Cursaro C, Gasbarrini G. Interferon-a increases prostaglandin E2 production by cultured liver biopsy in patients with chronic viral hepatitis: can non-steroidal anti-inflammatory drugs improve the therapeutic response to interferon? J Hepatol 1993; 19(2): 228–31
Huber BE, Wirth PJ, Newbold JE. Effects of human lympho-blastoid interferon on proliferation, gene expression and tumourigenicity of human hepatoma cell lines. Drugs Exp Clin Res 1991; 17: 281–91
Dianzani F, Antonelli G, Currenti M, et al. Induction of neutralizing antibodies to interferon during treatment of hepatitis patients: comparison among different interferon preparations [abstract]. Antiviral Res 1991 Apr; 15Suppl. 1: 127
Brand CM, Leadbeater L. Specificities of therapy-induced anti-interferon-α antibodies. J Interferon Res 1994 Aug; 14: 201–3
Antonelli G, Currenti M, Turriziani O, et al. Neutralizing antibodies to interferon-α: relative frequency in patients treated with different interferon preparations. J Infect Dis 1991 Apr; 163: 882–5
Brand CM, Leadbeater L, Bellati G, et al. Antibodies developing against a single recombinant interferon protein may neutralize many other interferon-α subtypes. J Interferon Res 1993 Apr; 13: 121–5
Dianzani F, Baron S. Medical microbiology. 3rd ed. New York: Churchill Livingstone, 1991
Samuel CE. Mechanisms of the antiviral action of the interferons. Prog Nucleic Acid Res Mol Biol 1988; 35: 27–72
Caselmann WH, Meyer M, Scholz S, et al. Type 1 interferons inhibit hepatitis B virus replication and induce hepatocellular gene expression in cultured liver cels. J Infect Dis 1992; 166: 966–71
Samuel CE. Antiviral actions of interferon. Interferon-regulated cellular proteins and their surprisingly selective antiviral activities. Virology 1991; 183: 1–11
Thomas NSB, Linch DC. An intracellular 50-kDa Fcψ-binding protein is induced in human cells by α-IFN. J Immunol 1991; 146(5): 1649–54
Hannigan GE, Williams BRG. Signal transduction by interferon-α through arachidonic metabolism. Science 1991; 251: 204–7
Andreone P, Cursaro C, Gramenzi A, et al. Indomethacin increases 2′,5′-oligoadenylate synthetase release by cultured liver tissue of patients with HCV chronic active hepatitis. Int Hepatol Commun 1994; 2(5): 289–94
Edman JC, Gray P, Valenzuela P, et al. Integration of hepatitis B virus sequences and their expression in a human hepatoma cell. Nature 1980 M 31; 286: 535–8
Popper H. The relation between hepatitis B virus infection and hepatocellular carcinoma. Hepatogastroenterology 1986; 33: 2–5
Chuang W-L, Chang W-Y, Lu S-N, et al. The role of hepatitis B and C viruses in hepatocellular carcinoma in a hepatitis B endemic area. A case-control study. Cancer 1992; 69: 2052–4
Colombo M, Choo QL, Del Ninno E, et al. Prevalence of antibody to hepatitis C virus in Italian patients with hepatocellular carcinoma. Lancet 1989; II: 1006–8
Simonetti RG, Cammà C, Fiorello F, et al. Hepatitis C virus infection as a risk factor for hepatocellulr carcinoma in patients with cirrhosis. A case-control study. Ann Intern Med 1992; 2: 97–102
Mazzella G, Accogli E, Sottili S, et al. Alpha interferon treatment may prevent hepatocellular carcinoma in HCV-related liver cirrhosis. J Hepatol 1996; 24(2): 141–7
Liaw Y-F. Current therapeutic trends for chronic viral hepatitis. J Gastroenterol Hepatol 1997; 12: S346–53
Oon J-C, Tan N, Lim GK. Natural lymphoblastoid Wellferon in the prevention of hepatocellular carcinoma in high-risk (HBsAg) positive carriers: A 5-year follow-up [abstract]. IXth Triennial International Symposium on viral hepatitis and liver disease; 1996 Apr 21–25; Rome, B32
Oon J-C. Long-term survival following treatment of hepatocellular carcinoma in Singapore: evaluation of Wellferon in the prophylaxis of high-risk pre-cancerous conditions. Cancer Chemother Pharmacol 1992; 31 Suppl.: S137–142
Shafritz DA, Shouval D, Sherman HI, et al. Integration of hepatitis B virus DNA into the genome of liver cells in chronic liver disease and hepatocellular carcinoma. N Engl J Med 1981; 305(18): 1067–73
Nishiguchi S, Kuroki T, Nakatani S, et al. Randomised trial of effects of interferon-α on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet 1995 Oct 21; 346: 1051–5
Bellentani S, Tiribelli C, Saccoccio G, et al. Prevalence of chronic liver disease in the general population of Northern Italy: The Dionysos Study. Hepatology 1994; 20: 1442–9
Antonelli G, Giannelli G, Currenti M, et al. Antibodies to interferon (IFN) in hepatitis C patients relapsing while continuing recombinant IFN-a2 therapy. Clin Exp Immunol 1996; 104: 384–7
Wong S, Gauthier T, Kaita KDE, et al. The differential effects of three forms of interferon alfa on hepatic regeneration after partial hepatectomy in the rat. Hepatology 1995 Sep; 22: 883–6
Hoofnagle JH, Di BAM. The treatment of chronic viral hepatitis. N Engl J Med 1997 Jan 30; 336: 347–56
Desmet V, Gerber M, Hoofnagle JH, et al. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology 1994; 19: 1513–20
Woo MH, Burnakis TG. Interferon alfa in the treatment of chronic viral hepatitis B and C. Ann Pharmacother 1997 Mar; 31(3): 330–7
Liaw Y-F, Lin S-M, Chen T-J, et al. Beneficial effect of prednisolone withdrawal followed by human lymphoblastoid interferon on the treatment of chronic type B hepatitis in Asians: a randomized controlled trial. J Hepatol 1994 Feb; 20: 175–80
Castilla A, Civeira MP, Jáuregui JL, et al. Treatment of chronic hepatitis B with lymphoblastoid alpha interferon [in Spanish]. Med Clin 1989; 93(16): 601–3
Fattovich G, Farci P, Rugge M, et al. A randomized controlled trial of lymphoblastoid interferon-αlpha in patients with chronic hepatitis-B lacking HBeAg. Hepatology 1992 Apr; 15: 584–9
Saracco G, Mazzella G, Rosina F, et al. A controlled trial of human lymphoblastoid interferon in chronic hepatitis B in Italy. Hepatology 1989; 10(3): 336–41
Wong DKH, Yim C, Naylor CD, et al. Interferon alfa treatment of chronic hepatitis B: randomized trial in a predominantly homosexual male population. Gastroenterology 1995 Jan; 108: 165–71
Catterall AP, King R, Lau JYN, et al. Interferon-α therapy with and without interferon-α priming in patients with chronic hepatitis B infection. J Antimicrob Chemother 1993; 31(5): 777–82
Fattovich G, Giustina G, Brollo L, et al. Therapy for chronic hepatitis B with lymphoblastoid interferon-α and levamisole. Hepatology 1992 Nov; 16: 1115–9
Janssen HLA, Berk L, Heijtink RA. Interferon-α and zidovudine combination therapy for chronic hepatitis B: results of a randomized, placebo-controlled trial. Hepatology 1993 Mar; 17: 383–8
Zavaglia C, Botelli R, Bellati G, et al. Treatment of chronic hepatitis B (HBeAg-HBV DNA-positive) with lymphoblastoid alpha interferon with or without corticosteroids: short-and long-term follow-up. Ital J Gastroenterol 1996; 28(6): 324–31
Cianciara J, Gladysz A, Juszczyk J, et al. Randomized controlled trial of alpha-interferon (Wellferon) alone or following prednisone withdrawal in chronic hepatitis B — results of a multicenter, double blind study [in Polish]. Pol Arch Med Wewn 1992 Dec; 88: 430–40
Buti M, Jardi R, Rodriguez-Frias F, et al. Interferon vs. adenine arabinoside 5′-monophosphate in patients with anti-HBe-positive chronic hepatitis. J Med Virol 1996; 49(4): 325–8
Wong DKH, Cheung AM, O’Rourke K, et al. Effect of alpha-interferon treatment in patients with hepatitis B e antigen-positive chronic hepatitis B: a meta-analysis. Ann Intern Med 1993; 119: 312–23
Korenman J, Baker B, Waggoner J, et al. Long-term remission of chronic hepatitis B after alpha-interferon therapy. Ann Intern Med 1991; 114(8): 629–34
Scullard GH, Smith CI, Merigan TC, et al. Effects of immuno-suppressive therapy on viral markers in chronic active hepatitis B. Gastroenterology 1981; 81: 987–91
Sagnelli E, Manzillo G, Maio G, et al. Serum levels of hepatitis B surface and core antigens during immunosuppressive treatment of HB sAg-positive chronic active hepatitis. Lancet 1980 Aug 23; II: 395–7
Weiler IVD, Bassendine MF, Murray A, et al. The effects of prednisolone/azathioprine in chronic hepatitis B viral infection. Gut 1982: 650–5
Cohard M, Poynard T, Mathurin P, et al. Prednisone-interferon combination in the treatment of chronic hepatitis B: direct and indirect metanalysis. Hepatology 1994 Dec; 20: 1390–8
Kuo G, Choo Q-L, Alter HJ, et al. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science 1989; 244: 362–4
Alter MJ. Hepatitis C: a sleeping giant? Am J Med 1991; 91Suppl. 3B: 112–5S
Alter MJ. Epidemiology of hepatitis C. Hepatology 1997; 26Suppl. 1: 62S–5S
Dienstag J. Sexual and perinatal transmission of hepatitis C. Hepatology 1997; 26(3) Suppl. 1: 66S–70S
Lindsay KL. Therapy of hepatitis C: overview. Hepatology 1997; 26Suppl. 1: 71S–7S
Craxì A, Di Marco V, Iacono OL, et al. Transfusion-associated chronic hepatitis C: alpha-n1 interferon for 6 vs. 12 months. J Hepatol 1996 May; 24: 539–46
Camps J, Castilla A, Ruiz J, et al. Randomised trial of lymphoblastoid α-interferon in chronic hepatitis C. Effects on inflammation, fibrogenesis and viremia. J Hepatol 1993; 17(3): 390–6
De Alava E, Camps J, Pardo-Mindan J, et al. Histological outcome of chronic hepatitis C treated with a 12-month course of lymphoblastoid alfa interferon. Liver 1993; 13(2): 73–9
Rumi M, Del Ninno E, Parravicini ML, et al. A prospective, randomized trial comparing lymphoblastoid to recombinant interferon alfa 2a as therapy for chronic hepatitis C. Hepatology 1996 Dec; 24: 1366–70
Tanaka K, Kondo M, Sakaguchi T, et al. Efficacy of ursodeoxycholic acid in combination with interferon-α in treating chronic hepatitis C: results of a long-term follow-up trial. J Gastroenterol Hepatol 1996 Dec; 11: 1155–60
Marcellin P, Hopf U, Trepo C, et al. A randomised double-blind, controlled multicentre study of lymphoblastoid interferon alpha N1 in the treatment of adults with chronic hepatitis C. Glaxo Wellcome Inc. 1997 (Data on file)
Farrell GC, Bacon BR, Goldin RD, et al. Lymphoblastoid interferon-αn1 improves the long-term response to a six-month course of treatment in chronic hepatitis C compared with recombinant interferon-α2b. Results of an international ran- domized controlled trial. Glaxo Wellcome Inc. 1997 (Data on file)
Kagawa T, Morizane T, Saito H, et al. A randomized, controlled trial of weekly administration of lymphoblastoid interferon in patients with chronic hepatitis C. J Hepatol 1993; 17(1): 91–6
Taltavull TC, Baliellas C, Sese E, et al. Interferon may be useful in hemodialysis patients with hepatitis C virus chronic infection who are candidates for kidney transplant. Transplant Proc 1995; 27(4): 2229–30
Rasi G, DiVirgilio D, Mutchnick MG, et al. Combination thymosin α1 and lymphoblastoid interferon treatment in chronic hepatitis C. Gut 1996 Nov; 39: 679–83
Kallinowski B, Hofmann WJ, Otto HF, et al. Therapy of chronic hepatitis C and non-A, non-B with alpha -interferon [in German]. Inn Med 1991; 18(4): 80–3
Tine F, Magrin S, Craxi A, et al. Interferon for non-A, non-B chronic hepatitis. A meta-analysis of randomised clinical trials. J Hepatol 1991; 13: 192–9
Bardelli F, Messori A, Rampazzo R, et al. Effect of recombinant or lymphoblastoid interferon-α on alanine aminotransferase in patients with chronic hepatitis C or chronic non-A non-B hepatitis. Clin Drug Invest 1995; 9(5): 239–54
Poynard T, Leroy V, Cohard M, et al. Meta-analysis of interferon randomized trials in the treatment of viral hepatitis C: effects of dose and duration. Hepatology 1996; 24(4): 778–89
Bacon BR, Farrell G, Benhamou JP Lymphoblastoid interferon improves long-term response to a six month course of treatment when compared with recombinant interferon alfa 2b: results of an international trial [abstract]. Hepatology 1995 Oct; 22 (Pt. 2): 152
Bellati G, Roffi L, Colloredo G, et al. Breakthrough and interferon dose in the therapy of chronic hepatitis C [abstract]. Hepatology 1996 Jan; 23: 1–41
Antonelli G, Giannelli G, Currenti M, et al. Fate of antibody to interferon (IFN) in chronic hepatitis C patients treated with recombinant IFN alpha 2 and retreated with lymphoblastoid IFN alpha. Int Hepatol Commun 1995 Dec; 4: 232–7
Milella M, Antonelli G, Santantonio T, et al. Treatment with natural IFN of hepatitis C patients with or without antibodies to recombinant IFN. Hepatogastroenterology 1995; 42(3): 201–4
Bellati G, Colloredo MG, Roffi L, et al. Prevalence of breakthrough during alpha interferon therapy for chronic hepatitis C [abstract]. Hepatology 1994 Oct; 20 (Pt 2): 331A
Colloredo G, Bellati G, Roffi L, et al. Prevalence of breakthrough in chronic hepatitis C during alpha interferon therapy [abstract]. J Gastroenterol Hepatol 1994 May-Jun; 9: A41
Bellati G, Colloredo G, Roffi L, et al. Prevalence of breakthrough in chronic hepatitis C patients undergoing antiviral therapy: role of type and schedule of alpha interferon. J Hepatol 1997 Feb; 26: 449–50
Roffi L, Colloredo Mels G, Antonelli G, et al. Breakthrough during recombinant interferon alfa therapy in patients with chronic hepatitis C virus infection: prevalence, etiology and management. Hepatology 1995; 21Suppl. 3: 645–9
Cimino L, Citarella C, Nardone G, et al. Lymphoblastoid interferon in chronic hepatitis C patients, ‘non-responders’ to recombinant interferon-α (rIFN-α). J Hepatol 1992; 14(2-3): 419–20
Ajello A, Freni MA, Spadaro A, et al. Lymphoblastoid interferon (IFN) in anti-HCV positive chronic hepatitis unaffected by other interferons [abstract]. J Hepatol 1993; 18Suppl. 1: S87–8
Sachs E, Di Bisceglie AM, Dusheiko GM, et al. Treatment of hepatocellular carcinomas with recombinant leukocyte interferon: A pilot study. Br J Cancer 1985; 52: 105–9
Lyons SF, Schoub BD, Kew MC, et al. The use of interferon functional assays in the laboratory monitoring of patients with hepatocellular carcinoma receiving interferon. Am J Clin Pathol 1986; 85: 450–5
Saracco G, Rizzetto M. The long-term efficacy of interferon alfa in chronic hepatitis C patients: a critical review. J Gastroenterol Hepatol 1995 Nov-Dec; 10: 668–73
Camps J, Garcia-Granero M, Riezu-Boj JI, et al. Prediction of sustained remission of chronic hepatitis C after a 12-month course of alfa interferon. J Hepatol 1994; 21(1): 4–11
Martinot-Peignoux M, Marcellin P, Pouteau M, et al. Pretreatment serum hepatitis C virus RNA levels and hepatitis C virus genotype are the main and independent prognostic factors of sustained response to interferon alfa therapy in chronic hepatitis C. Hepatology 1995; 22(4): 1050–6
Hermida M, Castro A, Calvo SL, et al. HCV genotypes and viremia in predicting response to interferon alfa in chronic hepatitis C [abstract]. Hepatology 1995 Oct; 22 Pt 2: 428A
Davis GL, Lau JYN. Factors predictive of a beneficial response to therapy of hepatitis C. Hepatology 1997; 26Suppl. 1: 122S–7S
Thomas HC, Scully LJ, McDonald JA. Lymphoblastoid and recombinant α-A interferon therapy of chronic hepatitis B virus infection. The Royal Free Hospital experience. J Hepatol 1986; 3Suppl. (5) 2: S193–7
Hopf U, Berg T, König V, et al. Treatment of chronic hepatitis C with interferon alpha: long-term follow-up and prognostic relevance of HCV genotypes. J Hepatol 1996; 24Suppl. 2: 67–73
Camps J, Crisóstomo S, Garcia-Granero M, et al. Prediction of the response of chronic hepatitis C to interferon alfa: a statistical analysis of pretreatment variables. Gut 1993; 34(12): 1714–7
Pagliaro L, Craxi A, Cammaa C, et al. Interferon- alpha for chronic hepatitis C: an analysis of pretreatment clinical predictors of response. Hepatology 1994; 19(4): 820–8
Fargion S, Fracanzani AL, Sampietro M, et al. Liver iron influences the response to interferon alpha therapy in chronic hepatitis C. Eur J Gastroenterol Hepatol 1997; 9: 497–503
Scully LJ, Brown D, Lloyd C, et al. Immunological studies before and during interferon therapy in chronic HBV infection: identification of factors predicting response. Hepatology 1990; 12(5): 1111–7
Guadagnino V, Caroleo B, Izzi A, et al. T-lymphocyte sub-populations as factors predicting clinical response to interferon in hepatitis C virus-related chronic active hepatitis [2]. Infection 1995; 23(3): 189–90
Mazzella G, Salzetta A, Casanova S, et al. Treatment of chronic sporadic-type non-A, non-B hepatitis with lymphoblastoid interferon: gamma GT levels predictive for response. Dig Dis Sci 1994; 39(4): 866–70
Yoshioka K, Yano M, Terazawa Y, et al. Randomized controlled trial of lymphoblastoid interferon α for chronic hepatitis C (comparions of 6 MU versus 9 MU dosages): early disappearance of HCV RNA is essential for sustained response. American Association for the Study of Liver Diseases. Postgraduate Courses and 48th Annual Meeting. 1997 Nov 7–11; Chicago, 421A
Gil B, Qian C, Riezu-Boj JI, et al. Hepatic and extrahepatic HCV RNA strands in chronic hepatitis C: different patterns of response to interferon treatment. Hepatology 1993; 18(5): 1050–4
Pardo M, Marriott E, Moliner MC, et al. Risks and benefits of interferon-α in the treatment of hepatitis. Drug Saf 1995 Nov; 13: 304–16
Marinho R, Serejo F, Raimundo M, et al. Adverse effects in hepatitis B and C treated with interferon. Any difference? [abstract]. Hepatology 1996 Oct; 24 Pt 2: Program Suppl.: 543
Mazzella G, Villanova N, Abdu-Ahmed M, et al. Treatment of chronic hepatitis B with human lymphoblastoid interferon: results of a controlled trial [in English]. Chemioterapia 1988 Dec; 7Suppl. 3: 12–4
Janssen HLA, Berk L, Schalm SW, et al. Antiviral effect of prolonged intermittent lymphoblastoid alpha interferon treatment in chronic hepatitis B. Gut 1992 Aug; 33: 1094–8
Haria M, Benfield P. Interferon-α-2a: a review of its pharmacological properties and therapeutic use in the management of viral hepatitis. Drugs 1995 Nov; 50: 873–96
Shakil AO, Di Bisceglie-AM, Hoofnagle JH. Seizures during alpha interferon therapy. J Hepatol 1996; 24(1): 48–51
Di Cesare E, Previti M, Russo F, et al. Interferon-α therapy may induce insulin autoantibody development in patients with chronic viral hepatitis. Dig Dis Sci 1996; 41(8): 1672–7
Marcellin P, Pouteau M, Benhamou J-P. Hepatitis C virus infection, alpha interferon therapy and thyroid dysfunction. J Hepatol 1995 Mar; 22: 364–9
Lisker-Melman M, Di Bisceglie AM, Usala SJ, et al. Development of thyroid disease during therapy of chronic viral hepatitis with interferon alfa. Gastroenterology 1992; 102(6): 2155–60
Custro N, Montalto G, Scafidi V, et al. Prospective study on thyroid autoimmunity and dysfunction related to chronic hepatitis C and interferon therapy. J Endocrinol Invest 1997; 20: 374–80
Sonnante AM, Cozzolongo R, Ragnini F, et al. Organ specific autoantibodies in patients with chronic hepatitis treated with interferon [abstract]. Gut 1995; 37Suppl. 2: A234–235
Marazuela M, Garcia-Buey L, Gonzalez-Fernandez B, et al. Thyroid autoimmune disorders in patients with chronic hepatitis C before and during interferon-α therapy. Clin Endocrinol 1996; 44(6): 635–42
Perillo RP. Interferon in the management of chronic hepatitis B. Dig Dis Sci 1993; 38: 577–93
National Institutes of Health. National Institutes of Health Consensus Development Conference Panel Statement: management of hepatitis C. Hepatology 1997; 26(3) Suppl. 1: 2S–10S
Schalm SW, Fattovich G, Brouwer JT. Therapy of hepatitis C: patients with cirrhosis. Hepatology 1997; 26(3): 128S–32S
Alter MJ, Mast EE. The epidemiology of viral hepatitis in the United States. Gastroenterol Clin N Am 1994; 23: 437–55
Moradpour D, Wands JR. Understanding hepatitis B infection. N Engl J Med 1995; 332: 1092–3
McCarron B, Main J, Thomas HC. Viral hepatitis, new viruses and therapies. Curr Opin Infect Dis 1996 Oct; 9: 359–64
Di Bisceglie AM, Goodman ZD, Ishak KG, et al. Long-term clinical and histopathological follow-up of chronic posttransfusion hepatitis. Hepatology 1991; 14: 969–74
Tong MJ, El-Farra NS, et al. Clinical outocmes after transfusion-associated hepatitis. N Engl J Med 1995; 332: 1464–6
Alter HJ. Descartes before the horse: I clone, therefore I am: the hepatitis C virus in current perspective. Ann Intern Med 1991; 115: 644–9
Lemon SM, Thomas DL. Vaccines to prevent viral hepatitis. N Engl J Med 1997 Jan 16; 336: 196–203
Dusheiko GM, Khakoo S, Soni P, et al. A rational approach to the management of hepatitis C infection. BMJ 1996 Feb 10; 312: 357–64
Cantell K. Development of antiviral therapy with alpha interferons: promises, false hopes and accomplishments. Ann Med 1995 Feb; 27: 23–8
Saracco G, Rizzetto M. A practical guide to the use of interferons in the management of hepatitis virus infections. Drugs 1997 Jan; 53: 74–85
Dusheiko GM. New treatments for chronic viral hepatitis B and C. Baillieres Clin Gastroenterol 1996 Jul; 10: 299–333
Böker KH, Ringe B, Krüger M, et al. Prostaglandin E plus famciclovir — a new concept for the treatment of severe hepatitis B after liver transplantation. Transplantation 1994; 57: 1706–8
Doong S-L, Tsai C-H, Schinazi RF, et al. Inhibition of the replication of hepatitis B virus in vitro by 2′3′-dideoxy-3′-thiacytidine and related analogues. Proc Natl Acad Sci USA 1991; 88: 8495–9
Dienstag JL, Perrillo RP, Schiff ER, et al. A preliminary trial of lamivudine for chronic hepatitis B infection. N Engl J Med 1995; 333: 1657–61
Dienstag JL, Schiff ER, Gitlin N, et al. Extended lamivudine retreatment for chronic hepatitis B. Hepatology 1996; 24 Suppl. 188A
Kania RJ, Friedlander L, Van Thiel DH, et al. Lamivudine plus interferon for the treatment of IFN-nonresponsive chronic hepatitis C infection. American Association for the study of Liver Diseases; Postgraduate Courses and 48th Annual Meeting [abstract]; 1997 Nov 7–11; Chicago, 1626.
Thomas HC, Booth J, Brown J. Pathophysiology and treatment of hepatitis C. Drugs 1996; 52Suppl. 2: 1–8
Alexopoulou A, Papakonstantinou A, Zafiropoulou E, et al. Randomised trial in HBeAg negative patients with replicating virus with lamivudine or ganciclovir in combination with interferon. Preliminary data. Hepatology 26 (Suppl.): Pt 2, Oct 1997
Blair CS, Haydon GH, Hayes PC. Current perspectives on the treatment and prevention of hepatitis C infection. Expert Opin Invest Drug 1996 Dec; 5: 1657–71
Colquhoun SD. Hepatitis C: a clinical update. Arch Surg 1996 Jan; 131: 18–23
Gregorio GV, Jara P, Hierro L, et al. Lymphoblastoid interferon alfa with or without steroid pretreatment in children with chronic hepatitis B: a multicenter controlled trial. Hepatology 1996 Apr; 23: 700–7
Giacchino R, Facco F, Giambartolomei G, et al. Treatment of children with chronic hepatitis B with a combination of steroids and human lymphoblastoid interferon. Chemioterapia 1988 Dec; 7Suppl. 3: 20–5
Farci P, Karayiannis P, Brook MG, et al. Treatment of chronic hepatitis delta virus (HDV) infection with human lymphoblastoid alpha interferon. Q J Med 1989; 73(271): 1045–54
Marzano A, Ottobrelli A, Spezia C, et al. Treatment of early chronic delta hepatitis with lymphoblastoid alpha interferon: A pilot study. Ital J Gastroenterol 1992; 24(3): 119–21
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perry, C.M., Wagstaff, A.J. Interferon-α-n1. BioDrugs 9, 125–154 (1998). https://doi.org/10.2165/00063030-199809020-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00063030-199809020-00004