Abstract
Objective: To evaluate the efficacy and tolerability of different dosages of candesartan cilexetil administered as monotherapy or in combination with any pre-existing but insufficient antihypertensive treatment. The effects of candesartan cilexetil treatment on quality of life were also investigated.
Design and Setting: This was an open, multicentre, nonrandomised study conducted at 1575 centres throughout Germany.
Patients: 4531 patients with primary hypertension receiving no or insufficient antihypertensive treatment [diastolic blood pressure (DBP) >95mm Hg] participated in the study.
Interventions: Patients received candesartan cilexetil 8mg once daily in addition to any pre-existing antihypertensive therapy. The dosage was increased to 16mg after 4 weeks and to 32mg after 8 weeks if sitting clinic DBP remained ≥90mm Hg.
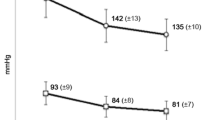
Results: Candesartan cilexetil (mean dose 14.8mg) reduced mean sitting DBP and systolic blood pressure by 16 (from 102 to 86mm Hg) and 26mm Hg (from 172 to 145mm Hg), respectively. Blood pressure control (DBP<90mm Hg) was achieved in 67.8% of patients by week 12. The mean Psychological General Well-Being index was improved by 9.2 units. Candesartan cilexetil was well tolerated with only 6.1% of patients reporting an adverse event.
Conclusions: This study demonstrates that treatment with candesartan cilexetil is efficacious and well tolerated in patients with mild to moderate primary hypertension. The efficacy of candesartan cilexetil is independent of antihypertensive co-medication, achieving a DBP close to 85mm Hg in most patients.
Similar content being viewed by others
References
Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet 1998; 351: 1755–62
Noda M, Shibouta Y, Inada Y, et al. Inhibition of rabbit aortic angiotensin II (All) receptor by CV-11974, a new nonpeptide All antagonist. Biochem Pharmacol 1993 Jul 20; 46: 311–8
Elmfeldt D, George M, Hübner R, et al. Candesartan cilexetil, a new generation angiotensin II antagonists, provides dose dependent antihypertensive effect. J Hum Hypertens 1997; 11 Suppl. 2: S49–54
Andersson OK, Neldam S. Candesartan cilexetil 16 mg provides greater antihypertensive effect than losartan 50 mg in patients with mild to moderate hypertension. Am J Hypertens 1997; 10: 125A
Malmqvist K, Swedish Multicentre Group. In hypertensive women, candesartan cilexetil lowers BP more effectively and is better tolerated than enalapril or a diuretic. Am J Hypertens 199; 12(Pt 2): 139
Farsang C, Kawecka-Jaszcz K, Langan J, on behalf of the CHAMP study group. Antihypertensive effects and tolerability of candesartan cilexetil, amlodipine and their combination. Am J Hypertens 1997; 10: 80A
Heuer HJ, Schöndorfer G, Högemann AM. Twenty-four hour blood pressure profile of different doses of candesartan cilexetil in patients with mild to moderate hypertension. J Hum Hypertens 1997; 25: 14–21
Meredith PA, Perloff D, Mancia G, et al. Blood pressure variability and its implications for antihypertensive therapy. Blood Press 1995; 4: 5–11
Lacourcière Y, Asmar R, Poirier L, on behalf of the CHAMP study group. Impact of missed doses of candesartan and losartan on 48-hour blood pressure control in ambulatory hypertensive patients. J Hypertens 1999; 17 Suppl. 3: S197
Belcher G, Hübner R, George M, et al. Candesartan cilexetil: safety and tolerability in healthy volunteers and patients with hypertension. J Hum Hypertens 1997; 11 Suppl. 2: S49–54
Bloom BS. Continuation of initial antihypertensive medication after 1 year of therapy. Clin Ther 1998; 20(4): 671–81
Franke H, Hermann WM, Magin SG. Comparison of the efficacy and safety of candesartan cilexetil 4, 8 and 12 mg with placebo and enalapril 10 mg in patients with mild to moderate essential hypertension. Am J Hyp 1997; 10: 90A
Sever P, Holzgreve H. Long term efficacy and tolerability of candesartan cilexetil in patients with mild to moderate hypertension. J Hum Hypertens 1997; 11 Suppl. 2: S69–73
Zanchetti A, Omboni S, DiBiago C, et al. Candesartan cilexetil and enalapril are of equivalent efficacy in patients with mild to moderate hypertension. J Hum Hypertens 1997; 11 Suppl. 2: S57–59
Adler CH, Sethi KD, Hauser RA, et al. Ropinirole for the treatment of early Parkinson’s disease. Neurology 1997; 49(2): 393–9
Campion GV, Lebsack ME, Lookabaugh J, et al. Dose-range and dose-frequency study of recombinant human interleukin-1 receptor antagonist in patients with rheumatoid arthritis. Arthritis Rheum 1996; 39(7): 1092–101
Rapaport M, Coccaro E, Sheline Y, et al. A comparison of fluvoxamine and fluoxetine in the treatment of major depression. J Clin Psychopharmacol 1996; 16(5): 373–8 Correspondence and reprints: Dr K-L Schulte, Evangelisches Krankenhaus Königin Elisabeth, Akademisches Lehrkrankenhaus der Charité, Gefäβzentrum Berlin/ Medizinische Klinik Herzbergstrasse 79, D-10362 Berlin, Germany. E-mail: k.schulte@keh-berlin.de
Author information
Authors and Affiliations
Corresponding author
Additional information
Atacand Under Real-Life Aspects.
Rights and permissions
About this article
Cite this article
Schulte, K.L., Fischer, M., Lenz, T. et al. Efficacy and Tolerability of Candesartan Cilexetil Monotherapy or in Combination with Other Antihypertensive Drugs. Clin. Drug Investig. 18, 453–460 (1999). https://doi.org/10.2165/00044011-199918060-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-199918060-00004