Summary
Abstract
Etanercept (Enbrel®), which inhibits the activity of tumour necrosis factor-α, is indicated in the treatment of patients with active rheumatoid arthritis (RA). A lifetime cost-utility analysis in patients with severe disease-modifying antirheumatic drug (DMARD)-resistant RA in the UK suggested that etanercept is associated with acceptable cost-utility ratios relative to traditional nonbiological DMARDs. In a 12-month cost-utility study in Spain, etanercept was predicted to be dominant over infliximab plus methotrexate in patients with active, refractory RA with regards to the cost per QALY gained and cost per American College of Rheumatology (ACR) 20 response achieved. In short-term cost-effectiveness analyses conducted in the US, the cost effectiveness of etanercept relative to other treatments in patients with methotrexate-naive or -resistant RA depends on whether predicted incremental cost-effectiveness ratios of at least $US41 900 per ACR 20 response or $US34 800 per ACR 70 weighted response over a 6-month period are considered acceptable (1999 values).
The relative efficacy and cost effectiveness of etanercept and other biological DMARDs will be clarified when appropriate data from directly comparative clinical and/or long-term pharmacoeconomic studies become available. Etanercept may prevent or delay disability, which may produce reductions in nondrug costs that could help offset its acquisition cost.
Rheumatoid Arthritis
RA, a chronic autoimmune disease, is characterised by symmetrical joint swelling and tenderness, progressive joint damage, pain, loss of function, disability and premature mortality. Substantial direct, indirect and intangible costs, which increase as the severity of the disease increases, are associated with RA. Early aggressive treatment of RA with DMARDs may prevent or delay disease progression.
Clinical Profile
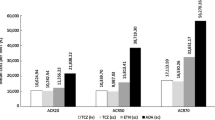
In well designed clinical trials and their extension phases, subcutaneous etanercept 25mg twice weekly produced rapid, sustainable clinical responses in methotrexate-naive or DMARD-resistant patients with RA. Etanercept was generally associated with significantly higher ACR 20, 50 and 70 response rates during the first 6 months of treatment, slower radiographic progression and more rapid improvement in measures of health-related quality of life (HR-QOL) than methotrexate in a 1-year trial in methotrexate-naive patients with early RA. In 6-month trials in DMARD-resistant patients, a significantly greater proportion of patients achieved ACR 20, 50 and 70 responses at 3 and 6 months with etanercept monotherapy or etanercept plus methotrexate than with placebo or methotrexate plus placebo. Other measures of disease activity also improved to a significantly greater extent with etanercept than with the comparators.
Most adverse events associated with etanercept in clinical trials were of mild-to-moderate severity. Approximately one-third of patients experienced mild-to-moderate injection site reactions. Infections, which occurred at a similar frequency with etanercept and placebo, were the most common serious adverse event.
Pharmacoeconomic Analyses
In patients with DMARD-resistant RA, a lifetime cost-utility model from the perspective of a healthcare payer in the UK predicted that third-line etanercept is cost effective compared with current care (a sequence of intramuscular gold, leflunomide and ciclosporin plus methotrexate as third-, fourth and fifth-line therapy). The incremental cost per QALY gained of etanercept followed by current care relative to current care alone was £16 330 (year 2000 values). The etanercept sequence was associated with higher drug and monitoring costs, but lower costs for other healthcare interventions, and greater effectiveness and QALYs gained. When loss-of-productivity and long-term care costs were included in sensitivity analysis, the incremental cost per QALY gained decreased to <£8000.
From the perspective of society, etanercept was predicted to be dominant over infliximab plus methotrexate with regards to cost per QALYs gained and cost per ACR 20 response achieved in a modelled 12-month cost-utility analysis in patients with active, refractory RA in Spain.
US cost-effectiveness analyses have modelled events during the first 6 months of various treatments in patients with RA and have predicted incremental cost-effectiveness ratios (ICERs) as the additional cost per ACR 20 response or ACR 70 weighted (70W) response relative to the next-best nondominated option. When total costs (direct plus loss-of-productivity costs) were considered in methotrex ate-naive patients, etanercept was more effective, but also more costly, than leflunomide, methotrexate.15 mg/week, sulfasalazine or no treatment; predicted ICERs of etanercept relative to other treatments were $US41 900 per ACR 20 response and $US40 800 per ACR 70W response over 6 months (1999 values). In methotrexate-resistant patients, etanercept plus methotrexate was more effective, but was also more costly than etanercept monotherapy, ciclosporin plus methotrexate, triple therapy (hydroxychloroquine, sulfasalazine and methotrexate), continuation of methotrexate therapy or no second-line treatment when total costs were considered. Predicted ICERs of etanercept relative to other treatments were $US42 600 per ACR 20 response and $US34 800 per ACR 70W response over 6 months. Etanercept monotherapy was both more effective and more costly than triple therapy for both ACR outcomes. When only direct costs were considered, the ICERs of etanercept were slightly higher.
In cost-minimisation analyses from a healthcare payer or societal perspective in The Netherlands or the US, the total per-patient annual costs associated with etanercept were estimated to be less than those associated with infliximab.







Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Immunex Corporation. Enbrel (etanercept) US prescribing information. Thousand Oaks (CA), 2003 Nov 25
Pearce GJ, Chikanza IC. Targeting tumour necrosis factor in the treatment of rheumatoid arthritis. Biodrugs 2001; 15 (3): 13949
European Medicines Agency. Enbrel (etanercept): summary of product characteristics [online]. Available from URL: http:// www.emea.eu.int [Accessed 2004 Apr 26]
American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines. Guidelines for the management of rheumatoid arthritis: 2002 update. Arthritis Rheum 2002 Feb; 46 (2): 328–46
Kvien TK. Epidemiology and burden of illness of rheumatoid arthritis. Pharmacoeconomics 2004; 22 Suppl. 1: 1–12
Lubeck DP. Patient-reported outcomes and their role in the assessment of rheumatoid arthritis. Pharmacoeconomics 2004; 22 Suppl. 1: 27–38
Symmons DP. Epidemiology of rheumatic arthritis: determinants of onset, persistence and outcome. Best Pract Res Clin Rheumatol 2002 Dec; 16 (5): 707–22
Clarke AE, St Pierre Y, Joseph L, et al. Radiographic damage in rheumatoid arthritis correlates with functional disability but not direct medical costs. J Rheumatol 2001; 28 (11): 2416–24
Wolfe F, Freundlich B, Straus WL. Increase in cardiovascular and cerebrovascular disease prevalence in rheumatoid arthritis. J Rheumatol 2003; 30 (1): 36–40
Wolfe F, Michaud K, Gefeller O, et al. Predicting mortality in patients with rheumatoid arthritis. Arthritis Rheum 2003 Jun; 48 (6: 1530–42
Felson DT, Anderson JJ, Boers M, et al. American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995; 38 (6: 727–35
Fries IF, Spitz P, Kraines RG, et al. Measurement of patient outcome in arthritis. Arthritis Rheum 1980 Feb; 23 (2): 137–45
Sharp IT, Young DY, Bluhm GB, et al. How many joints in the hands and wrists should be included in a score of radiologic abnormalities used to assess rheumatoid arthritis? Arthritis Rheum 1985 Dec; 28 (12): 1326–35
National Institute for Clinical Excellence. Guidance on the use of etanercept and infliximab for the treatment of rheumatoid arthritis. London: National Institute for Clinical Excellence, 2002 Mar. Technology appraisal guidance: no. 36
Cooper NJ. Economic burden of rheumatoid arthritis: a systematic review. Rheumatology (Oxford) 2000; 39 (1): 28–33
Pugner KM, Scott DI, Holmes JW, et al. The costs of rheumatoid arthritis: an international long-term view. Semin Arthritis Rheum 2000 Apr; 29 (5): 305–20
Lubeck DP. A review of the direct costs of rheumatoid arthritis: managed care versus fee-for-service settings. Pharmacoeconomics 2001; 19 (8): 811–8
Michaud K, Messer J, Choi HK, et al. Direct medical costs and their predictors in patients with rheumatoid arthritis: a threeyear study of 7,527 patients. Arthritis Rheum 2003 Oct; 48 (10): 2750–62
van Jaarsveld CH, Jacobs JW, Schrijvers AJ, et al. Direct cost of rheumatoid arthritis during the first six years: a cost-of-illness study. Br J Rheumatol 1998; 37 (8): 837–47
Merkesdal S, Ruof J, Schoffski O, et al. Indirect medical costs in early rheumatoid arthritis: composition of and changes in indirect costs within the first three years of disease. Arthritis Rheum 2001 Mar; 44 (3): 528–34
Young A, Dixey J, Kulinskaya E, et al. Which patients stop working because of rheumatoid arthritis? Results of five years’ follow up in 732 patients from the Early RA Study (ERAS). Ann Rheum Dis 2002; 61 (4): 335–40
Albers JMC, Kuper HH, van Riel PLCM, et al. Socio-economic consequences of rheumatoid arthritis in the first years of the disease. Rheumatology (Oxford) 1999; 38: 423–30
Kosinski M, Kujawski SC, Martin R, et al. Health-related quality of life in early rheumatoid arthritis: impact of disease and treatment response. Am J Manag Care 2002 Mar; 8 (3): 231–40
Yelin E, Wanke LA. An assessment of the annual and long-term direct costs of rheumatoid arthritis. Arthritis Rheum 1999 Jun; 42 (6: 1209–18
Kavanaugh A, Han C, Bala M. Functional status and radiographic joint damage are associated with health economic outcomes in patients with rheumatoid arthritis. J Rheumatol 2004; 31 (5): 849–55
das Chaga Mederios MM, Ferraz MB, Quaresma MR. The effect of rheumatoid arthritis on the quality of life of primary caregivers. J Rheumatol 2000; 27 (1): 76–83
Moreland LW, Baumgartner SW, Schiff MH, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med 1997 Jul 17; 337 (3): 141–7
Bathon JM, Martin RW, Fleischmann RM, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med 2000 Nov 30; 343 (22): 1586–93
Moreland LW, Schiff MIL Baumgartner SW, et al. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med 1999 Mar 16; 130 (6): 478–86
Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor: Fe fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med 1999 Jan 28; 340 (4): 253–9
Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet 2004 Feb 28; 363 (9410): 675–81
Culy CR, Keating GM. Etanercept: an updated review of its use in rheumatoid arthritis, psoriatic arthritis and juvenile rheumatoid arthritis. Drugs 2002; 62 (17): 2493–537
Keating GM, Jarvis B. Management of rheumatoid arthritis: defining the role of etanercept. Dis Manage Health Outcomes 2002; 10 (1): 17–39
Fleischmann R, Iqbal I, Nandeshwar P, et al. Safety and efficacy of disease-modifying anti-rheumatic agents: focus on the benefits and risks of etanercept. Drug Saf 2002; 25 (3): 173–97
Genovese MC, Martin RW, Fleischmann RM, et al. Etanercept (Enbrel) in early erosive rheumatoid arthritis (ERA trial): clinical and radiographic data [abstract no. 208]. Arthritis Rheum 2003; 48 (9 Suppl.): S122–123
Moreland LW, Cohen SB, Klareskog L, et al. Global safety and efficacy of more than 5 years of etanercept (Enbrel) therapy in rheumatoid arthritis [abstract no. 215]. Arthritis Rheum 2003 Sep; 28 (9 Suppl.): S125
Klareskog L, van de Heijde D, De Jager J, et al. Clinical outcomes of a double-blind study of etanercept and methotrexate, alone and combined, in patients with active rheumatoid arthritis (TEMPO trial), year 2 results [abstract no. OP0003]. Ann Rheum Dis 2004 Jul; 63 Suppl. 1: 58–9
Genovese MC, Bathon JM, Martin RW, et al. Etanercept versus methotrexate in patients with early rheumatoid arthritis: twoyear radiographic and clinical outcomes. Arthritis Rheum 2002 Jun; 46 (6: 1443–50
Hochberg MC, Chang RW, Dwosh I, et al. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum 1992 May; 35 (5): 498–502
Moreland LW, Cohen SB, Baumgartner SW, et al. Longterm safety and efficacy of etanercept in patients with rheumatoid arthritis. J Rheumatol 2001; 28 (6): 1238–44
Kremer JM, Weinblatt ME, Bankhurst AD, et al. Etanercept added to background methotrexate therapy in patients with rheumatoid arthritis: continued observations. Arthritis Rheum 2003 Jun; 48 (6: 1493–9
Mathias SD, Colwell HH, Miller DP, et al. Health-related quality of life and functional status of patients with rheumatoid arthritis randomly assigned to receive etanercept or placebo. Clin Ther 2000 Jan; 22 (1): 128–39
Yelin E, Trupin L, Katz P, et al. Association between etanercept use and employment outcomes among patients with rheumatoid arthritis. Arthritis Rheum 2003 Nov; 48 (11): 3046–54
Moreland LW, Bucy RP, Weinblatt ME, et al. Immune function in patients with rheumatoid arthritis treated with etanercept. Clin Immunol 2002 Apr; 103 (1): 13–21
Furst DE, Breedveld FC, Kalden JR, et al. Updated consensus statement on biological agents for the treatment of rheumatoid arthritis and other immune mediated inflammatory diseases (May 2003). Ann Rheum Dis 2003; 62 Suppl. 2: ii2–9
Brennan A, Bansback N, Reynolds A, et al. Modelling the costeffectiveness of etanercept in adults with rheumatoid arthritis in the UK. Rheumatology (Oxford) 2004; 43 (1): 62–72
Hernández-Cruz B, Rubio-Terrés C, Ariza-Ariza R, et al. Evaluación económica de etanercept frente a infiiximab-metotrexato en el tratamiento de al artritis reumatoide resistente a fármacos antirreumáticos modificadores de la enfermedad. Pharmacoeconomics Sp Res Art 2004; 1 (2): 73–85
Choi HK, Seeger JD, Kuntz KM. A cost effectiveness analysis of treatment options for methotrexate-naive rheumatoid arthritis. J Rheumatol 2002; 29 (6: 1156–65
Choi HK, Seeger JD, Kuntz KM. A cost-effectiveness analysis of treatment options for patients with methotrexate-resistant rheumatoid arthritis. Arthritis Rheum 2000 Oct; 43 (10): 231627
Nuijten MJ, Engelfriet P, Duijn K, et al. A cost-cost study comparing etanercept with infiiximab in rheumatoid arthritis. Pharmacoeconomics 2001; 19 (10): 1051–64
Ollendorf DA, Peterson AN, Doyle J, et al. Impact of leflunomide versus biologic agents on the costs of care for rheumatoid arthritis in a managed care population. Am J Manag Care 2002 May; 8 (7 Suppl.): S203–13
Kobelt G, Eberhardt K, Geborek P. TNF inhibitors in the treatment of rheumatoid arthritis in clinical practice: costs and outcomes in a follow up study of patients with RA treated with etanercept or infiiximab in southern Sweden. Ann Rheum Dis 2004; 63 (1): 4–10
British Society for Rheumatology. Guidelines for prescribing TNF-alpha blockers in adults with rheumatoid arthritis: report of a Working Party of the British Society for Rheumatology. London: The British Society for Rheumatology, 2001 Apr 2
Anderson JJ, Wells G, Verhoeven AC, et al. Factors predicting response to treatment in rheumatoid arthritis. Arthritis Rheum 2000 Jan; 43 (1): 22–9
Kobelt G, Eberhardt K, Jönsson L, et al. Economic consequences of the progression of rheumatoid arthritis in Sweden. Arthritis Rheum 1999 Feb; 42 (2): 347–56
Jobanputra P, Barton P, Bryan S, et al. The effectiveness of infliximab and etanercept for the treatment of rheumatoid arthritis: a systematic review and economic evaluation. Health Technol Assess 2002; 6 (21): 1–110
Maim R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. Lancet 1999 Dec 4; 354: 1932–9
Wolfe F, Michaud K, Pincus T. Do rheumatology cost-effectiveness analyses make sense? Rheumatology (Oxford) 2004; 43 (1): 4–6
Brennan A, Bansback N. Re: Wolfe et al. Do rheumatology cost-effectiveness analyses make sense? [letter]. Rheumatology (Oxford) 2004; 43 (5): 677–8
Torrance GW, Tugwell P, Amorosi S, et al. Improvement in health utility among patients with rheumatoid arthritis treated with adalimumab (a human anti-TNF monoclonal antibody) plus methotrexate. Rheumatology (Oxford) 2004; 43 (6: 7128
Emery P. Review of health economics modelling in rheumatoid arthritis. Pharmacoeconomics 2004; 22 Suppl. 1: 55–69
Drug Facts & Comparisons [online]. Available from URL: http://www.efactsweb.com [Accessed 2004 May 4]
Baumgartner SW, Fleischmann RM, Moreland LW, et al. Etanercept (Enbrel) in patients with rheumatoid arthritis with recent onset versus established disease: improvement in disability. J Rheumatol 2004; 31 (8): 1532–7
Hochberg MC, Tracy JK, Hawkins-Holt M, et al. Comparison of the efficacy of the tumour necrosis factor alpha blocking agents adalimumab, etanercept, and infliximab when added to methotrexate in patients with active rheumatoid arthritis. Ann Rheum Dis 2003; 62 Suppl. 2: ii13–6
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lyseng-Williamson, K.A., Plosker, G.L. Etanercept. Pharmacoeconomics 22, 1071–1095 (2004). https://doi.org/10.2165/00019053-200422160-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-200422160-00004