Abstract
Abstract
Insulin lispro is a recombinant insulin analogue with transposed amino acids (proline and lysine) at positions 28 and 29 near the C-terminus of the B-chain. The most prominent practical advantage of insulin lispro over human soluble insulin lies in its very rapid onset of action. This property allows it to be injected immediately before meals and minimises the demands made on patients with type 1 diabetes mellitus, and those with type 2 disease who require insulin, by the ongoing need for careful meal planning and timing. Numerous clinical studies have shown significant improvements in postprandial glycaemic control, with some evidence of reduced rates of severe or nocturnal hypoglycaemia, relative to conventional human insulin in patients receiving lispro-based insulins
Quality-of-life studies show consistent preferences by patients for and increased treatment satisfaction with insulin lispro over human soluble insulin, particularly with variations of the Diabetes Treatment Satisfaction Questionnaire. Willingness of patients and taxpayers to pay additional costs for insulin lispro or a premixed lispro-based formulation over conventional human insulins, and cost benefits favouring formulary inclusion, have been shown in well designed studies carried out in Australia and Canada. Spanish data suggest cost effectiveness in terms of episodes of severe hypoglycaemia avoided, and preliminary German resource utilisation data indicate cost savings related to reduced hospitalisation and general practice costs, with insulin lispro relative to human soluble insulin.
Conclusions: Insulin lispro and premixed formulations of lispro-based insulins offer quality-of-life improvements relative to conventional human insulins in patients with diabetes mellitus. Participants in well designed studies have expressed a preference for lispro-based insulins and have been shown to be willing to pay for the advantages they offer, and current cost-benefit data favour the inclusion of these insulins in formularies and their reimbursement by third party payers. Further research into the pharmacoeconomic implications of insulin lispro use in the long termis needed, particularly with respect to effects on indirect costs and those associated with complications of diabetes mellitus.
Cost of Illness, Glycaemic Control and Quality of Life in Patients with Diabetes Mellitus
Diabetes mellitus accounts for approximately 8% of total healthcare budgets across developed countries, a figure that is set to increase substantially alongside the global prevalence of this disease during the first quarter of the 21st century (from 4% in 1995 to an estimated 5.4% in 2025)
The cost to healthcare systems of diabetes mellitus is considerable. Comprehensive analyses carried out from a government perspective for the years 1995 and 1997 have shown the annual direct cost of diabetes mellitus in the US to be around $US45 billion. This figure increased to $US98 billion when indirect costs were included for 1997. Two-thirds of all costs were incurred by persons aged 65 years and over. Mean total per capita expenditures in 1997 were $US10 071 for patients with diabetes mellitus and $US2669 for persons without the disease. The direct medical costs associated with type 2 diabetes mellitus were estimated in eight European countries in a study involving more than 7000 patients. The total direct cost of type 2 diabetes mellitus in the eight countries was 29 billion euros annually or 2834 euros per patient per year (1999 values); hospitalisations accounted for 55% of direct costs. In another analysis, production losses attributable to morbidity and premature mortality accounted for 57% of the total annual cost of diabetes mellitus in Sweden in 1994 of 5746 million Swedish kronor (≈$US747 million).
Research published over the past decade has shown an association between improvements in long-term glycaemic control and reduced incidence of microvascular complications in patients with diabetes mellitus. US figures show the annual cost of intensive management aimed at tighter glycaemic control to be two to three times that of conventional therapy in patients with type 1 disease, but results are nevertheless available to indicate that sustained reductions in levels of glycated haemoglobin (HbA1c) are associated with overall healthcare cost savings within 1 to 2 years of improvement.
The importance of quality-of-life considerations in patients with diabetes mellitus has been underlined in particular by a utility value analysis in 292 persons that indicated a willingness on the part of the average participant to trade 12% of remaining life expectancy for freedom from diabetes mellitus. Furthermore, a self-management survey in 2056 respondents showed significantly (p < 0.01) lower quality of life on physical and social functioning scales of the short form (SF)-20 questionnaire in patients with insulin-treated type 2 diabetes mellitus than in those with type 1 diabetes mellitus or those with type 2 diabetes mellitus managed with diet and oral antihyperglycaemic drugs.
Clinical Profile of Insulin Lispro
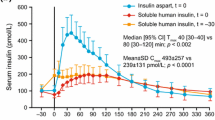
Randomised clinical studies, most of which were of nonblind and crossover design, have generally shown similar overall long-term glycaemic control (as shown by mean HbA1c values) and fasting blood glucose levels in patients receiving insulin lispro (from 0 to 15 minutes before meals) and those being treated with human soluble insulin (20 to 45 minutes before meals). However, small but statistically significant reductions in mean HbA1c values relative to human soluble or isophane insulins, respectively, have been reported in some studies of insulin lispro or premixed insulin lispro (25%) and neutral protamine lispro (75%) [Mix25].1
Mean 2-hour postprandial blood glucose levels were significantly (p < 0.05) lower with insulin lispro than with human soluble insulin (either in addition to basal intermediate- or long-acting insulin) in the majority of clinical studies of intensive multiple-dose therapy. In addition, patients receiving insulin lispro showed reductions in postprandial blood glucose levels or increases that were significantly (p < 0.05) smaller than with human soluble insulin. A small study in 20 patients with type 1 diabetes mellitus who received insulin lispro for at least 1 year (mean 1.8 years) has indicated long-term maintenance of superiority of insulin lispro over human soluble insulin in terms of postprandial glycaemic profiles. Data from two larger trials also demonstrated better glycaemic control with insulin lispro than human soluble insulin over a 1-year period.
There were no increases relative to human insulins in overall rates of hypoglycaemia in patients receiving lispro-based insulins, with lower incidences of severe or nocturnal hypoglycaemia being noted in some trials.
Two nonblind randomised trials in a total of 314 patients with type 2 diabetes mellitus showed that 4 months of treatment with twice-daily administration of Mix25 achieved greater glycaemic control than glibenclamide (glyburide) 15 mg/day. The incidence of hypoglycaemiawas, however, higher in patients treated with the premixed lispro formulation.
The combination of insulin lispro immediately before meals with glibenclamide 10mg twice daily was associated with decreased mean HbA1c levels relative to bedtime human isophane insulin plus glibenclamide in two parallel-group studies in 554 patients with type 2 diabetes mellitus and secondary sulphonylurea failure. Both trials also indicated significantly (p ≤ 0.05) better postprandial glycaemic control with preprandial insulin lispro plus bedtime human isophane insulin or twice-daily glibenclamide than with glibenclamide plus bedtime human isophane insulin or metformin 1 to 2.5g daily.
Results obtained in children receiving insulin lispro show similar activity relative to human soluble insulin to that noted in adults, and three reports in 246 pregnant women with type 1 diabetes mellitus have linked insulin lispro to improved glycaemic control and reduced risk of hypoglycaemia relative to human soluble insulin in this patient group.
Studies in patients receiving continuous subcutaneous insulin infusion showed significantly (p = 0.047 to 0.004) lower mean HbA1c levels over 2 to 4 months with insulin lispro than with human soluble insulin, with no overall differences in rate of hypoglycaemia. Two-hour postprandial blood glucose levels were consistently lower in patients receiving insulin lispro.
A meta-analysis of eight multicentre studies in 2576 patients with type 1 diabetes mellitus has shown a reduction in overall frequency of severe hypoglycaemia with insulin lispro relative to human soluble insulin. Pooled analysis of ten clinical studies in 3634 patients with type 1 or type 2 diabetes mellitus has shown similar incidences of treatment-emergent adverse events or progression of retinopathy, neuropathy or cardiovascular disease with insulin lispro and human soluble insulin.
Pharmacoeconomic Considerations
Quality of Life in Patients Receiving Insulin Lispro. Increased satisfaction with treatment and improved quality of life with insulin lispro relative to other insulin therapies have been shown in a number of studies with quality of life as a primary or secondary endpoint in patients with diabetes mellitus
Clear preferences for insulin lispro over human soluble insulin, as shown by the Diabetes Treatment Satisfaction Questionnaire (DTSQ), revised Questionnaire on Stress in Diabetes and the Diabetes Quality-of-Life Measure, were noted in three nonblind studies (reported as abstracts) after 284 to 2216 patients with type 1 or type 2 diabetes mellitus were transferred from the latter treatment to the former. In one study in 598 patients, 87.5% expressed a preference for insulin lispro on the grounds of increased freedom with meal timing and increased independence in everyday and physical activities.
Similar findings have been reported in randomised comparisons. DTSQ scores from a Japanese 24-week parallel-group study in 426 patients (preliminary report) showed greater treatment satisfaction (p = 0.023) and reduced frequency of undesirable hypoglycaemia (p < 0.001) with insulin lispro relative to human soluble insulin. A fully reported nonblind crossover study in 52 patients with a history of successful treatment with insulin showed superiority of insulin lispro over human soluble insulin with the DTSQ in its original eight-item form (p = 0.0008) and an extended 20-item version (DTSQ-S) [p = 0.0001] for total satisfaction and all categories of meal-related and correctional use of insulin. A further version of the DTSQ (DTSQ-C), designed for sensitivity to effects of changes in therapy, showed particular improvement (all p = 0.0001 vs human soluble insulin) in satisfaction with current treatment, convenience, flexibility, understanding and controllability of blood glucose levels, and wish to continue with current treatment; preferences not evident with the DTSQ-S (recommendation of treatment to others, predictability of blood glucose levels and recognition/correction of mild hypoglycaemia; p ≤ 0.01) were also noted with the DTSQ-C. No differences between treatments were shown by the Well-Being Questionnaire in this study.
Results from clinical trials with quality of life as a secondary endpoint mostly showed higher levels of treatment satisfaction with insulin lispro than with human soluble insulin. Analysis of results from two nonblind, crossover studies in a total of 942 evaluable patients with type 1 or type 2 diabetes mellitus showed greater (p ≤ 0.001) increases from baseline in mean Diabetes Quality of Life Clinical Trial Questionnaire scores after 3 months for mean treatment flexibility (3.1 vs 0.8) and treatment satisfaction (4.7 vs 0.4) with insulin lispro than with human soluble insulin in patients with type 1 diabetes mellitus.
Willingness to Pay for Insulin Lispro. Well designed and fully reported willingness-to-pay (WTP) analyses have been carried out from the perspectives of Australian patients with diabetes mellitus (insulin lispro vs human soluble insulin; n = 83) and Canadian taxpayers [Mix25 vs premixed 30% human soluble and 70% isophane insulins (human 30/70); n = 80]. Significantly higher proportions of respondents preferred insulin lispro or Mix25 over conventional human insulins in both studies. Australian cost-benefit data showed an incremental benefit of insulin lispro over human soluble insulin (mean WTP) of 37.68 Australian dollars ($A)/month (≈$US23.36/month), with the overall WTP favouring insulin lispro (p < 0.0001) [year of costing not stated; conversion to US dollars at 30 June for year of study publication].
The net annual cost benefit of insulin lispro was $A381.84/patient (≈$US236.74/patient), which indicated that the drug should be considered for inclusion in Australian formularies. The net mean monthly WTP value for Mix25 was 35.28 Canadian dollars ($Can) for 1999 (≈$US23.99). The net annual cost benefit of $Can255 (≈$US173.40) indicated eligibility for consideration for formulary inclusion.
Additional data (abstract available) from 69 Australian patients with type 2 diabetes mellitus showed an overall monthly WTP for Mix25 over human 30/70 of $A95.62 (≈$US57.37) [year of costing not stated; conversion to US dollars at 30 June for year of study publication], with a total incremental benefit per patient of $A82.14/month (≈$US49.28/month).
Cost-Effectiveness and Other Studies. Two reports of Spanish cost-effectiveness studies in patients with type 1 diabetes mellitus are available. One of these (published as an abstract) showed, relative to human soluble insulin, a cost per episode of severe hypoglycaemia avoided by use of insulin lispro of $US60 per 100 patient-years on the basis of results of one clinical trial, and a cost reduction of approximately $US129 per episode avoided per 100 patient-years on the basis of results of another (year of costing not stated). The other cost-effectiveness analysis (30 patients participating in a nonblind, crossover study) showed excess costs per 1% reduction in HbA1c level relative to human soluble insulin of $US0.85 and $US0.34 per day of treatment for insulin lispro plus 15 to 20% isophane insulin and insulin lispro alone, respectively (conversion from Spanish pesetas at January 2000).
Retrospective 1-year resource utilisation data from a German database indicated an overall annual cost saving, linked to reduced hospitalisation and general practice treatment costs, of 468 Deutschmarks per patient (≈$US229) [from a statutory health insurer’s perspective] relative to human soluble insulin in patients receiving insulin lispro (year of costing and size of database not stated in the abstract available; conversion to US dollars at 30 June for year of study publication).












Similar content being viewed by others
References
Bhattacharyya A. Treatment of type 2 diabetes mellitus. Hospital Pharmacist 2001 Jan; 8: 10–6
Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 2001 Jul 21; 358: 221–9
International Diabetes Center. About diabetes: risk factors [online]. Available from URL: http://www.parknicollet.com/diabetes [Accessed 2002 Aug 29]
Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000 Aug 12; 321: 405–12
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998 Sep 12; 352 (9131): 837–53
Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993 Sep 30; 329 (14): 977–86
Wilde MI, McTavish D. Insulin lispro: a review of its pharmacological properties and therapeutic use in the management of diabetes mellitus. Drugs 1997; 54: 597–614
Kenny SJ, Aubert RE, Geiss LS. Prevalence and incidence of non-insulin-dependent diabetes. Harris MI, Cowie CC, Stern MP, et al. Diabetes in America (NIH publication no. 95-1468). 2nd ed. Bethesda: National Diabetes Data Group, National Institutes of Health, 1995: 47–68
Burden of diabetes in the UK. Scrip 1999 Feb 26; (2415): 4
King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care 1998 Sep; 21 (9): 1414–31
World Health Organization. Global burden of diabetes: WHO projects a 170% growth in the number of people with diabetes in developing countries by 2025 [online]. Available from URL: http://www.who.int/inf-pr-1998/en/pr98-63.html [Accessed 2001 Sep 14]
Pagano E, Brunetti M, Tediosi F, et al. Costs of diabetes: a methodological analysis of the literature. Pharmacoeconomics 1999; 15 (6): 583–95
Krop JS, Powe NR, Weller WE, et al. Patterns of expenditures and use of services among older adults with diabetes. Implications for the transition to capitated managed care. Diabetes Care 1998; 21: 747–52
Krop JS, Saudek CD, Weller WE, et al. Predicting expenditures for Medicare beneficiaries with diabetes. A prospective cohort study from 1994 to 1996. Diabetes Care 1999; 22: 1660–6
Henriksson F, Jönsson B. Diabetes: the cost of illness in Sweden. J Intern Med 1998; 244: 461–8
American Diabetes Association. Economic consequences of diabetes mellitus in the U.S. in 1997. Diabetes Care 1998; 21: 296–309
Amin SP, Mullins CD, Duncan BS, et al. Direct health care costs for treatment of diabetes mellitus and hypertension in an IPAbgroup-model HMO. Am J Health Syst Pharm 1999; 56: 1515–20
Dawson KG, Gomes D, Gerstein H, et al. The economic cost of diabetes in Canada, 1998. Diabetes Care 2002 Aug; 25 (8): 1303–7
Dawson KG, Gomes D, Blanchard JF, et al. The economic cost of diabetes in Canada, 1998 [abstract no. P118]. Diabetes Res Clin Pract 2000 Sep; 50 Suppl. 1: S7
Hodgson TA, Cohen AJ. Medical care expenditures for diabetes, its chronic complications, and its comorbidities. Prev Med 1999; 29: 173–86
Currie CJ, Kraus D, Morgan CL, et al. NHS acute sector expenditure for diabetes: the present, future, and excess in-patient cost of care. Diabet Med 1997; 14: 686–92
Garattini L, Tediosi F, Chiaffarino F, et al. The outpatient costs of diabetes care in Italian diabetes centers. Value Health 2001; 4 (3): 251–7
Jönsson B. Revealing the cost of type II diabetes in Europe. Diabetologia 2002; 45: S5–12
Rathmann W, Haastert B, Roseman JM, et al. Prescription drug use and costs among diabetic patients in primary health care practices in Germany. Diabetes Care 1998; 21: 389–97
Diabetes Control and Complications Trial Research Group. Resource utilization and costs of care in the Diabetes Control and Complications Trial. Diabetes Care 1995; 18 (11): 1468–78
Eastman RC, Javitt JC, Herman WH, et al. Model of complications of NIDDM. I. Model construction and assumptions. Diabetes Care 1997 May; 20 (5): 725–34
Eastman RC, Javitt JC, Herman WH, et al. Model of complications of NIDDM. II. Analysis of the health benefits and cost-effectiveness of treating NIDDM with the goal of normoglycemia. Diabetes Care 1997; 20 (5): 735–44
Diabetes Control and Complications Trial Research Group. Lifetime benefits and costs of intensive therapy as practiced in the Diabetes Control and Complications Trial. JAMA 1996; 276 (17): 1409–15
Ramsey SD, Newton K, Blough D, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 1999; 22: 382–7
Gilmer TP, O’Connor PJ, Manning WG, et al. The cost to health plans of poor glycemic control. Diabetes Care 1997; 20: 1847–53
Wagner EH, Sandhu N, Newton KM, et al. Effect of improved glycemic control on health care costs and utilization. JAMA 2001; 285: 182–9
Rubin RR, Peyrot M. Quality of life and diabetes. Diabetes Metab Res Rev 1999; 15: 205–18
Bourdel-Marchasson I, Dubroca B, Manciet G, et al. Prevalence of diabetes and effect on quality of life in older French living in the community: the PAQUID Epidemiological Survey. J Am Geriatr Soc 1997; 45: 295–301
Koopmanschap M. Coping with type II diabetes: the patient’s perspective. Diabetologia 2002; 45: S18–22
Gulliford MC, Mahabir D. Social inequalitites in morbidity from diabetes mellitus in public primary care clinics in Trinidad and Tobago. Soc Sci Med 1998; 46 (1): 137–44
Gulliford MC, Mahabir D. Relationship of health-related quality of life to symptom severity in diabetes mellitus: a study in Trinidad and Tobago. J Clin Epidemiol 1999; 52: 773–80
Brown GC, Brown MM, Sharma S, et al. Quality of life associated with diabetes mellitus in an adult population. J Diabetes Complications 2000; 14: 18–24
Glasgow RE, Ruggiero L, Eakin EG, et al. Quality of life and associated characteristics in a large national sample of adults with diabetes. Diabetes Care 1997; 20: 562–7
Gale EA. A randomized, controlled trial comparing insulin lispro with human soluble insulin in patients with Type 1 diabetes on intensified insulin therapy. UK Trial Group. Diabet Med 2000; 17: 209–14
Bastyr III EJ, Johnson ME, Trautmann ME, et al. Insulin lispro in the treatment of patients with type 2 diabetes mellitus after oral agent failure. Clin Ther 1999; 21: 1703–14
Reviriego J, Herz M, Roach P, et al. Humalog® Mix25™ twice daily improves glycemic control compared to NPH twice daily in patients with type 2 diabetes [abstract no. 1526-PO]. Diabetes 2000 May; 49 Suppl. 1: A363–4
Ross SA, Zinman B, Campos RV, et al. A comparative study of insulin lispro and human regular insulin in patients with type 2 diabetes mellitus and secondary failure of oral hypoglycemic agents. Clin Invest Med 2001 Dec; 24 (6): 292–8
Vignati L, Anderson Jr JH, Iversen PW. Efficacy of insulin lispro in combination with NPH human insulin twice per day in patients with insulin-dependent or nonbinsulin-dependent diabetes mellitus. Multicenter Insulin Lispro Study Group. Clin Ther 1997; 19: 1408–21
Roach P, Strack T, Arora V, et al. Improved glycaemic control with the use of self-prepared mixtures of insulin lispro and insulin lispro protamine suspension in patients with types 1 and 2 diabetes. Int J Clin Pract 2001; 55 (3): 177–82
Roach P, Trautmann M, Arora V, et al. Improved postprandial blood glucose control and reduced nocturnal hypoglycemia during treatment with two novel insulin lispro-protamine formulations, insulin lispro Mix25 and insulin lispro Mix50. Mix50 Study Group. Clin Ther 1999; 21: 523–34
Bastyr III EJ, Stuart CA, Brodows RG, et al. Therapy focused on lowering postprandial glucose, not fasting glucose, may be superior for lowering HbA1c. Diabetes Care 2000; 23: 1236–41
Anderson Jr JH, Brunelle RL, Koivisto VA, et al. Improved mealtime treatment of diabetes mellitus using an insulin analogue. Clin Ther 1997; 19 (1): 62–72
Valle D, Santoro D, Bates P, et al. Italian multicentre study of intensive therapy with insulin lispro in 1184 patients with Type 1 diabetes. Diabetes Nutr Metab 2001 Jun; 14 (3): 126–32
Annuzzi G, Del Prato S, Arcari R, et al. Preprandial combination of lispro and NPH insulin improves overall blood glucose control in type 1 diabetic patients: a multicenter randomized crossover trial. Nutr Metab Cardiovasc Dis 2001; 11: 168–75
Anderson Jr JH, Brunelle RL, Koivisto VA, et al. Reduction of postprandial hyperglycemia and frequency of hypoglycemia in IDDM patients on insulin-analog treatment. Diabetes 1997; 46: 265–70
Anderson Jr JH, Brunelle RL, Keohane P, et al. Mealtime treatment with insulin analog improves postprandial hyperglycemia and hypoglycemia in patients with nonbinsulin-dependent diabetes mellitus. Arch Intern Med 1997; 157: 1249–55
Holleman F, Schmitt H, Rottiers R, et al. Reduced frequency of severe hypoglycemia and coma in well-controlled IDDM patients treated with insulin lispro. Benelux-UK Insulin Lispro Study Group. Diabetes Care 1997; 20: 1827–32
Garg SK, Anderson JH, Perry SV, et al. Long-term efficacy of humalog in subjects with type 1 diabetes mellitus. Diabet Med 1999; 16: 384–7
Lalli C, Ciofetta M, Sindaco PD, et al. Long-term intensive treatment of type 1 diabetes with the short-acting insulin analog lispro in variable combination with NPH insulin at mealtime. Diabetes Care 1999; 22: 468–77
Heller SR, Amiel SA, Mansell P, et al. Effect of the fast-acting insulin analog lispro on the risk of nocturnal hypoglycemia during intensified insulin therapy. U.K. Lispro Study Group. Diabetes Care 1999; 22: 1607–11
Mozejko-Pastewka B, Sieradzki J, Roach P. Postprandial blood glucose excursions are smaller with Humalog® Mix25™ than with Humulin® 30/70 [abstract no. P245]. Diabetes Res Clin Pract 2000; 50 Suppl. 1: 45
Roach P, Yue L, Arora V. Improved postprandial glycemic control during treatment with Humalog Mix25, a novel protamine-based insulin lispro formulation. Humalog Mix25 Study Group. Diabetes Care 1999; 22: 1258–61
Provenzano C, Vero R, Oliva A, et al. Lispro insulin in type 1 diabetic patients on a Mediterranean or normal diet: a randomized, cross-over comparative study with regular insulin. Diab Nutr Metab 2001; 14: 133–9
Ferguson SC, Strachan MW, Janes JM, et al. Severe hypoglycaemia in patients with type 1 diabetes and impaired awareness of hypoglycaemia: a comparative study of insulin lispro and regular human insulin. Diabetes Metab Res Rev 2001; 17 (4): 285–91
Chase HP, Lockspeiser T, Peery B, et al. The impact of the Diabetes Control and Complications Trial and Humalog insulin on glycohemoglobin levels and severe hypoglycemia in type 1 diabetes. Diabetes Care 2001; 24 (3): 430–4
Roach, Koledova E, Metcalfe S, et al. Glycemic control with Humalog Mix25 in type 2 diabetes inadequately controlled with glyburide. Clin Ther 2001 Oct; 23 (10): 1732–44
Herz M, Sun B, Milicevic Z, et al. Comparative efficacy of preprandial or postprandial Humalog Mix75/25 versus glyburide in patients 60 to 80 years of age with type 2 diabetes mellitus. Clin Ther 2002 Jan; 24 (1): 73–86
Deeb LC, Holcombe JH, Brunelle R, et al. Insulin lispro lowers postprandial glucose in prepubertal children with diabetes. Pediatrics 2001 Nov; 108 (5): 1175–9
Tupola S, Komulainen J, Jääskeläinen J, et al. Post-prandial insulin lispro vs. human regular insulin in prepubertal children with type 1 diabetes mellitus. Diabet Med 2001 Aug; 18 (8): 654–8
Holcombe JH, Zalani S, Arora VK, et al. Comparison of insulin lispro with regular human insulin for the treatment of type 1 diabetes in adolescents. Clin Ther 2002 Apr; 24 (4): 629–38
Patmore JE, Masson EA, Brash PD, et al. Maternal outcome in type 1 diabetic pregnancy treated with insulin lispro (IL) [abstract no. 2275-PO]. Diabetes 2001 Jun; 50 Suppl. 2: A538
Garg SK, Anil S, Gottlieb P, et al. Better glycemic control and reduced need for caesarian sections with insulin lispro treated pregnancies in type 1 diabetes [abstract no. 1602-P]. Diabetes 2001 Jun; 50 Suppl. 2: A383
Masson EA, Patmore J, Brash PD, et al. Perinatal outcome in type 1 diabetic pregnancy treated with insulin lispro (IL) [abstract no. P58]. Diabet Med 2001 Apr; 18 Suppl. 2: 53
Garg S, Pennington M, Anderson J. Maternal and fetal outcomes between human regular and Humalog® insulin treated pregnancies in type 1 diabetes [abstract no. 922]. Diabetologia 1999 Aug; 42 Suppl. 1: 245
Alawi H. Use of insulin lispro in pregnant women [abstract no. 1595-P]. Diabetes 2001 Jun; 50 Suppl. 2: A381
Bhattacharyya A, Brown S, Hughes S, et al. Insulin lispro and regular insulin in pregnancy. Q J Med 2001; 94: 255–60
Kadiri A, Al-Nakhi A, El-Ghazali S, et al. Treatment of type 1 diabetes with insulin lispro during ramadan. Diabetes Metab 2001 Sep; 27 (4): 482–6
Akram J, De Verga V. Insulin lispro (Lys(B28), Pro(B29)) in the treatment of diabetes during the fasting month of Ramadan. Ramadan Study Group. Diabet Med 1999; 16: 861–6
Renner R, Pfützner A, Trautmann M, et al. Use of insulin lispro in continuous subcutaneous insulin infusion treatment. Results of a multicenter trial. German Humalog-CSII Study Group. Diabetes Care 1999; 22: 784–8
Zinman B, Tildesley H, Chiasson J-L, et al. Insulin lispro in CSII: results of a double-blind crossover study [published erratum appears in Diabetes 1997 Jul; 46 (7): 1239]. Diabetes 1997; 46: 440–3
Melki V, Renard E, Lassmann-Vague V, et al. Improvement of HbA1c and blood glucose stability in IDDM patients treated with lispro insulin analog in external pumps. Diabetes Care 1998; 21: 977–82
Johansson UB, Adamson UCK, Lins PES, et al. Improved blood glucose variability, HbA1c insuman Infusat and less insulin requirement in IDDM patients using insulin lispro in CSII. The Swedish Multicenter Lispro Insulin Study. Diabetes Metab 2000; 26: 192–6
Raskin P, Holcombe JH, Tamborlane WV, et al. A comparison of insulin lispro and buffered regular human insulin administered via continuous subcutaneous insulin infusion pump. J Diabetes Complications 2001; 15 (6): 295–300
Hanaire-Broutin H, Melki V, Bessieres-Lacombe S, et al. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens using insulin lispro in type 1 diabetic patients on intensified treatment: a randomized study. Diabetes Care 2000; 23: 1232–5
Tsui E, Barnie A, Ross S, et al. Intensive insulin therapy with insulin lispro: a randomized trial of continuous subcutaneous insulin infusion versus multiple daily insulin injection. Diabetes Care 2001 Oct; 24 (10): 1722–7
Brunelle RL, Llewelyn J, Anderson Jr JH, et al. Meta-analysis of the effect of insulin lispro on severe hypoglycemia in patients with type 1 diabetes. Diabetes Care 1998; 21: 1726–31
Glazer NB, Zalani S, Anderson Jr JH, et al. Safety of insulin lispro: pooled data from clinical trials. Am J Health Syst Pharm 1999; 56: 542–7
Brunelle RL, Anderson Jr JH, Babbey LE, et al. Use of insulin lispro (Humalog®) in the elderly provides comparable safety to that observed with regular human insulin: a meta-analysis of safety data from controlled clinical trials [abstract no. P246]. Diabetes Res Clin Pract 2000 Sep; 50 Suppl. 1: S45
Costa B, Arroyo J, Sabaté A. The economics of pharmacotherapy for diabetes mellitus. Pharmacoeconomics 1997; 11 (2): 139–58
Reviriego J, Millan M. Does insulin lispro modify patient’s satisfaction in real life? [abstract no. 1265] Diabetes 1998 May; 47 Suppl. 1: A327
Pfützner A, Lindner U, Trautmann ME, et al. Insulin lispro and quality of life — results from the German QoL study [abstract no. 1369]. Diabetes 1998 May; 47 Suppl. 1: 354
Szelachowska M, Sieradzki J, Zonenberg A, et al. The evaluation of quality of life of patients with type 1 or type 2 diabetes during treatment with Humalog® [abstract no. P186]. Diabetes Res Clin Pract 2000 Sep; 50 Suppl. 1: S28
Ishii H, Ohashi Y, Kuzuya T. Quality-of-life assessment of insulin lispro in Japanese clinical trials — comparison with regular human insulin [abstract no. 367-P]. Diabetes 2000 May; 49 Suppl. 1: 91
Howorka K, Pumprla J, Schlusche C, et al. Dealing with ceiling baseline treatment satisfaction level in patients with diabetes under flexible, functional insulin treatment: assessment of improvements in treatment satisfaction with a new insulin analogue. Qual Life Res 2000; 9 (8): 915–30
Kotsanos JG, Vignati L, Huster W, et al. Health-related quality-of-life results from multinational clinical trials of insulin lispro. Assessing benefits of a new diabetes therapy. Diabetes Care 1997; 20 (6): 948–58
Herschbach P, Duran G, Waadt S, et al. Psychometric properties of the Questionnaire on Stress in Patients with Diabetes — Revised (QSD-R). Health Psychol 1997; 16: 171–4
Diabetes Control and Complications Trial Research Group. Influence of intensive diabetes treatment on quality-of-life outcomes in the Diabetes Control and Complications Trial. Diabetes Care 1996; 19: 195–203
Shen W, Kotsanos JG, Huster WJ, et al. Development and validation of the Diabetes Quality of Life Clinical Trial Questionnaire. Med Care 1999; 37: AS45–66
Bradley C, Lewis KS. Measures of psychological well-being and treatment satisfaction developed from the responses of people with tablet-treated diabetes. Diabet Med 1990; 7: 445–51
Lewis KS, Bradley C, Knight G, et al. A measure of treatment satisfaction designed specifically for people with insulin-dependent diabetes. Diabet Med 1988; 5: 235–42
Polonsky WH. Understanding and assessing diabetes-specific quality of life. Diabetes Spectrum 2000; 13: 36–41
Kohlmann C-W, Küstner E, Schuler M, et al. Mehrdimensionale krankheitsspezifische kontrollüberzeugungen bei typ I-diabetes mellitus: bericht über die weiterentwicklung eines fragebogenverfahrens. Zeitschrift Für Gesundheitspsychologie 1993; 1: 219–33
Davey P, Grainger D, MacMillan J, et al. Economic evaluation of insulin lispro versus neutral (regular) insulin therapy using a willingness-to-pay approach. Pharmacoeconomics 1998; 13 (3): 347–58
Dranitsaris G, Longo CJ, Grossman LD. The economic value of a new insulin preparation, Humalog® Mix 25™: measured by a willingness-to-pay approach. Pharmacoeconomics 2000; 18 (3): 275–87
Birch S, Gafni A, O’Brien B. Willingness to pay and the valuation of programmes for the prevention and control of influenza. Pharmacoeconomics 1999; 16 Suppl. 1: 55–61
Johannesson M, Jönsson B. Economic evaluation in health care: is there a role for cost-benefit analysis? Health Policy 1991; 17: 1–23
Johannesson M, Johansson P-O, Jönsson B. Economic evaluation of drug therapy: a review of the contingent valuation method. Pharmacoeconomics 1992 May; 1 (5): 325–37
Davey P, Grainger D, MacMillan J, et al. Clinical outcomes with insulin lispro compared with human regular insulin: a meta-analysis. Clin Ther 1997; 19: 656–74
Davey P, Rajan N, Schultz M, et al. Patients value the clinical characteristics and convenience of Humalog® Mix25™: results of a willingness to pay study in patients with type 2 diabetes [abstract no. P749]. Diabetes Res Clin Pract 2000 Sep; 50 Suppl. 1: 192
Sacristan J, Gomis R, Maranes JP, et al. Cost-effectiveness of insulin lispro versus regular insulin in patients with type 1 diabetes, based on severe hypoglycemia occurrence [abstract no. 924-P]. Diabetes 2000 May; 49 Suppl. 1: A223
Kilburg A, Clouth J, Daniel D, et al. The financial effects of interface agreements for diabetes mellitus: socioeconomic relevance of intensive controlled insulin therapy with insulin lispro compared to regular human insulin. Value Health 2000; 3: 339–40
Costa Pinel B, Belmonte Serrano M, Páez Vives F, et al. Conversion of intensive therapy with rapid insulin to lispro insulin in type-1 diabetes: a cost-effectiveness assessment [in Spanish]. Rev Clin Esp 2001 Aug; 201 (8): 448–54
Costa B, Sabaté A, Estopa A, et al. Lispro or regular insulin for type 1 diabetes treatment with multiple injection therapy? A cost-effectiveness assessment [abstract no. P1293]. Diabetes Res Clin Pract 2000 Sep; 50 Suppl. 1: S322
Raskin P, Klaff L, Bergenstal R, et al. A 16-week comparison of the novel insulin analog insulin glargine (HOE 901) and NPH human insulin used with insulin lispro in patients with type 1 diabetes. Diabetes Care 2000; 23: 1666–71
McKeage K, Goa KL. Insulin glargine: a review of its therapeutic use as a long-acting agent for the management of type 1 and 2 diabetes mellitus. Drugs 2001; 61 (11): 1599–624
Author information
Authors and Affiliations
Corresponding author
Additional information
Mix25 is used throughout the manuscript as a ‘generic’ term for this combination. In the US, this combination is marketed as Humalog® Mix 75/25™, while in the UK it is marketed as Humalog® Mix25™. Trade names are provided here for identification purposes only and do not indicate product endorsement.
Rights and permissions
About this article
Cite this article
Dunn, C.J., Plosker, G.L. Insulin Lispro. Pharmacoeconomics 20, 989–1025 (2002). https://doi.org/10.2165/00019053-200220140-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-200220140-00004