Abstract
Multiple sclerosis (MS) is a devastating disease that can occur in early life, progressing to rapid disability and loss of physical, psychosocial and economic functioning, significantly affecting quality of life. The traditional treatment for MS has been symptomatic, treating acute relapses without affecting the underlying disease. The introduction of interferon-β (IFNβ) has offered significant clinical benefits by reducing the frequency of relapses and slowing disease progression. Although the costs of this treatment are high, the costs to society of caring for a patient disabled by MS are greater, and if IFNβ can delay disease progression in the longer term, the economic impact would be substantial.
Previous pharmacoeconomic studies of IFNβ have suggested that benefits can only be achieved at extremely high cost, with reported cost-effectiveness measures of up to 1 million pounds sterling (£) per quality-adjusted life year (QALY) [1995 values]. However, these studies have considered only the short term benefits of IFNβ treatment: over 2 to 3 years, the impact of treatment on patients’ quality of life is relatively small, and cost-utility analyses that do not consider longer term benefits nor include societal costs may be misleading.
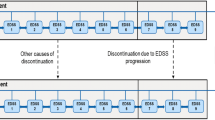
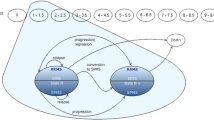
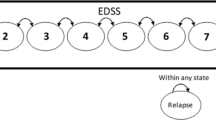
The model reported here is based on the hypothesis that the delay in disease progression seen in short term clinical trials is likely to continue if treatment is continued. The model also assumes that the delay in disease progression, which represents a reduction in brain atrophy, will result in lasting clinical benefits even if treatment is stopped. These assumptions are strongly supported by clinical trial data and the treatment hypothesis itself. A delay in disease progression will result in a significant improvement in functioning and quality of life, and if the costs associated with increased disability can be postponed, even long term treatment of MS with IFNβ can be shown to be cost effective.
Using resource utilisation costs derived from an economic evaluation of MS in the UK, it was possible to calculate the impact of delaying disease progression in terms of both health service and societal costs.
An estimate of mean disease progression in patients with MS treated with IFNβ-1a compared with patients who did not receive disease-modifying agents suggested that significant cost savings would be realised after about 12 years’ treatment with IFNβ-1a. The application of utility scores to the disease progression curves also facilitated estimates of cost effectiveness, with cost per QALY values ranging from £27 036 after 2 years’ treatment with IFNβ-1a to £37 845 after 20 years’ treatment (1995 values).










Similar content being viewed by others
References
Nicholson T, Milne R. Beta interferons (1a and 1b) in relapsing- remitting and secondary progressive multiple sclerosis. Development and Evaluation Committee Report No. 98. Southampton: Wessex Institute for Health Research and Development, 1999
Parkin D, McNamee P, Jacoby A, et al. A cost-utility analysis of interferon beta for multiple sclerosis. Health Technol Assessment 1998; 2 (4): iii-54
Murphy N, Confavreux C, Haas J, et al. Economic evaluation of multiple sclerosis in the UK, Germany and France. Pharmacoeconomics 1998; 13: 607–22
Forbes RB, Lees A, Waugh W, et al. Population based cost utility study of interferon beta-1b in secondary progressive multiple sclerosis. BMJ 1999; 319: 1529–33
Arnason BG, Dayal A, Qu ZX, et al. Mechanism of action of interferon- ß in multiple sclerosis. Semin Immunopathol 1996; 18: 125–48
Trapp BD, Ransohoff RM, Fisher E, et al. Neurodegeneration in multiple sclerosis: relationship to neurological disability. Neuroscientist 1999; 5: 48–57
Bradley WG. Recent views on amyotrophic lateral sclerosis with emphasis on electrophysiological studies. Muscle Nerve 1998; 10: 490–502
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–52
Weinshenker BG, Bass B, Rice GPA, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain 1989; 112: 133–46
Rudick RA, Fisher E, Lee J-C, et al. The impact of interferon beta-1a on progressive brain atrophy in patients with relapsing multiple sclerosis. Neurology 1999; 53: 1698–704
Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis: the Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol 1996; 39: 285–94
Whetten-Goldstein K, Sloan FA, Goldstein LB, etal. A comprehensive assessment of the cost of multiple sclerosis in the United States. Mult Scler 1998; 4: 419–25
Asche CV, Ho E, Chan B, et al. Economic consequences of multiple sclerosis for Canadians. Acta Neurol Scand 1997; 95: 268–74
Holmes J, Madgwick T, Bates D. The cost of multiple sclerosis. Br J Med Econ 1995; 8: 181–93
Bourdette DN, Prochazka AV, Mitchell W, et al. Health care costs of veterans with multiple sclerosis: implications for the rehabilitation of MS. VA MS Rehabilitation Study Group. Arch Phys Med Rehabil 1993; 74: 26–31
EUROQOL Group. EuroQol: a new facility for the measurement of health related quality of life. Health Policy 1990; 16: 199–208
Liu C, Blumhardt LD. Randomised, double blind, placebo controlled study of interferon ß-1a in relapsing-remitting multiple sclerosis analysed by area under disability/time curves. J Neurol Neurosurg Psychiatry 1999; 67: 451–6
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kendrick, M., Johnson, K.I. Long Term Treatment of Multiple Sclerosis with Interferon-ß May Be Cost Effective. Pharmacoeconomics 18, 45–53 (2000). https://doi.org/10.2165/00019053-200018010-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-200018010-00005