Summary
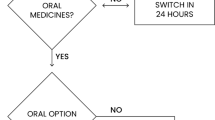
A few antibiotics (i.e. metronidazole, clindamycin and ciprofloxacin) are available in both parenteral and oral formulations, and have good bioavailability, ensuring equivalent systemic drug concentrations. During a 4-year period subsequent to the initiation of a parenteral to oral (IV-PO) stepdown programme for metronidazole and clindamycin, Vancouver General Hospital saved approximately $C85 000. However, many parenteral antibacterials lack an oral formulation, requiring oral stepdown to a different antibacterial with a similar spectrum of activity. Alternatively, the oral formulation of a parenteral antibacterial may have poor bioavailability (i.e. cefuroxime axetil, ampicillin, cloxacillin, erythromycin, and tetracycline) and it is not possible to maintain equivalent systemic drug concentrations. While rigid criteria are not applicable to all clinical scenarios, the general criteria for oral stepdown include the following: the patient 1) continues to need an antibiotic; 2) is clinically stable; 3) is capable of tolerating the oral dosage form; and 4) has no factors present (e.g. gastrointestinal abnormalities or drug interactions) that would adversely affect oral bioavailability. A review of subsequent IV-PO stepdown programmes at Vancouver General Hospital revealed that 1) not all patients receiving parenteral therapy are candidates for oral stepdown; 2) oral stepdown is delayed in a large proportion of treatment courses; 3) oral stepdown is not occurring in many patients for whom it is deemed appropriate; and 4) in a very few treatment courses stepdown may occur prematurely and may contribute to clinical deterioration. In 1991, acquisition costs for ceftriaxone, ceftazidime, and imipenem amounted to $C810 000, which represented 34% of total antibacterial drug expenses at Vancouver General Hospital. Cefixime, the first oral third generation cephalosporin marketed in Canada, can be used for oral stepdown in selected patients receiving ceftriaxone, ceftazidime, or ceftizoxime, resulting in decreased acquisition and delivery costs. Cefixime has been shown to be effective in the treatment of urinary tract infections, upper and lower respiratory tract infections, bacterial sinusitis and otitis media caused by susceptible pathogens; however, inadequate cerebrospinal fluid penetration precludes its use for the treatment of meningitis, and it lacks dependable activity against Pseudomonas aeruginosa, Staphylococcus aureus, and anaerobes. When applied judiciously, IV-PO stepdown can dramatically impact upon drug, drug delivery, and hospitalisation costs, lessen the incidence of IV-associated complications (e.g. phlebitis, infection), and facilitate early discharge.
Similar content being viewed by others
References
Bachand RL, Jewesson PJ, Chow AW. Implementation of a reserved antimicrobial drug program. Canadian Journal of Hospital Pharmacy: 40 167–170, 1987
Baldwin DR, Andrews JM, Ashby JP, et al. Concentrations of cefixime in bronchial mucosa and sputum. Letter. Thorax 45: 982, 1990a
Baldwin DR, Andrews JM, Ashby JP, et al. Concentrations of cefixime in bronchial mucosa and sputum after three oral multiple dosing regimens. Thorax 45: 401–402, 1990b
Block SL, Hedrick JA, Tyler RD. Comparative study of the effectiveness of cefixime and penicillin V for the treatment of streptococcal pharyngitis in children and adolescents. Pediatric Infectious Diseases Journal 11: 919–925, 1992
Brogden RN, Campoli-Richards DM. Cefixime: a review of its antibacterial activity, pharmacokinetic properties and therapeutic potential. Drugs 38: 524–550, 1989
Bunz D, Frighetto L, Gupta S, Jewesson P. Simple ways to promote cost containment. Drug Intelligence and Clinical Pharmacy, Annals of Pharmacotherapy 24: 546, 1990a
Bunz D, Gupta S, Jewesson PJ. Physician and nurse awareness of drug-use-review activities. American Journal of Hospital Pharmacy 46: 1125–1126, 1989
Bunz D, Gupta S, Jewesson P. Metronidazole cost containment: a two-stage intervention. Hospital Formulary 25: 1167–1177, 1990b
Faulkner RD, Bohaychuk W, Lane RA. Pharmacokinetics of cefixime in the young and elderly. Journal of Antimicrobial Chemotherapy 21: 787–794, 1988
Frighetto L, Bunz D, Martinusen S, Mamdani F, Jewesson P. IV-PO stepdown program: results of four years of experience. Annals of Pharmacotherapy 26: 1447–1451, 1992
Guay DRP, Meatherhall RC, Harding GK. Pharmacokinetics of cefixime (CL 284,635;FK 027) in healthy subjects and patients with renal insufficiency. Antimicrobial Agents and Chemotherapy 30: 485–490, 1986
Gupta S, Bachand R, Jewesson P. Unique two-stage intervention to modify prescribing trends. Drug Intelligence and Clinical Pharmacy 22: 726–727, 1988
Gupta S, Bachand R, Jewesson P. Impact of a two-stage intervention on cefazolin usage at a major teaching hospital. Hospital Formulary 24: 41–46, 1989
Gutensohn A, Bunz D, Frighetto L, Jewesson P. Outcome of a ceftriaxone/cefotaxime interchange programme in a major teaching hospital. Chemotherapy 37 (Suppl. 3): 15–21, 1991
Harrison CJ, Chartrand SA, Pichichero ME. Microbiologic and clinical aspects of a trial of once daily cefixime compared with twice daily cefaclor for treatment of acute otitis media in infants and children. Pediatric Infectious Diseases Journal 12: 62–69, 1993
Health Services Directorate. Health Services and Promotion Branch and Bureau of Communicable Disease Epidemiology. Health Protection Branch. Guidelines for antimicrobial utilization in health care facilities. Department of National Health and Welfare, p. 1–21, Ottawa, 1990
Iravani A, Richard GA, Johnson D. A double-blind, multicenter, comparative study of the safety and efficacy of cefixime versus amoxicillin in the treatment of acute urinary tract infections in adult patients. American Journal of Medicine 85 (Suppl. 3A): 17–23, 1988
Jewesson PJ, Bachand R, Chow AW. Evaluation of parenteral metronidazole in an acute care teaching hospital. Canadian Medical Association Journal 132: 785–789, 1985
Jewesson PJ, Chow AW. Auditing antibiotic use in a teaching hospital: focus on cefoxitin. Canadian Medical Association Journal 128: 1075–1078, 1983a
Jewesson PJ, Chow AW. Dealing with the misuse of antibiotics in the hospital. Canadian Medical Association Journal 128: 1061–1062, 1983b
Jewesson P, Chow A, Wai A, Frighetto L, Nickoloff D, et al. Double-blind comparison of cefoxitin (CFX), ceftizoxime (CTZ) and metronidazole-gentamicin (M-G) as prophylaxis for colorectal procedures. Abstract no. 533. 33rd ICAAC, New Orleans, Louisiana, October 17–20, 1993b
Jewesson PJ, Frighetto L, Mamdani F, Martinusen S. Recognizing and controlling the costs of IV antimicrobial therapy. Nosocomial infections: optimizing therapy while containing costs. Abstract. Royal College of Physicians and Surgeons of Canada 61st Annual Meeting, Ottawa, Canada, September 13, 1992
Jewesson P, Stiver G, Wai A, Frighetto L, Nickoloff D, et al. Double-blind comparison of cefazolin (CFX) and ceftizoxime (CTZ) for prophylaxis against infections following elective biliary tract surgery. Abstract no. 540. 33rd ICAAC, New Orleans, Louisiana, October 17–20, 1993a
Kiani R, Johnson D, Nelson B. Comparative multi-center studies of cefixime and amoxicillin in the treatment of respiratory tract infections. American Journal of Medicine 85 (Suppl. 3A): 6–13, 1988
Kunin CM. Problems in antibiotic usage. In Mundell et al. (Eds) Principles and practice of of infectious diseases, 3rd ed. pp. 427–434, Churchill Livingstone, Edinburgh, 1990
Laboratory Centre for Disease Control. Health Protection Branch, Department of National Health and Welfare. Canadian guidelines for the prevention, diagnosis, management and treatment of sexually transmitted diseases in neonates, children, adolescents and adults, Vol. 18, pp. 81–90, 1992
Leggett NJ, Caravaggio C, Rybak MJ. Cefixime. Annals of Pharmacotherapy 24: 489–494, 1990
Maesen FPV, Costongs R, Davies BJ. Concentrations of cefixime in bronchial mucosa and sputum. Letter. Thorax 45: 982, 1990
Martinusen S, Chen D, Frighetto L, Bunz D, Stiver G, et al. A comparison of cefoxitin and ceftizoxime in a hospital therapeutic interchange program. Canadian Medical Association Journal 148: 1161–1169, 1993
Matthews BL, Kohut RI, Edelstein DR, et al. Evaluation of cefixime in the treatment of bacterial maxillary sinusitis. Southern Medical Journal 86: 329–333, 1993
McLinn SF. Randomized, open label, multicenter trial of cefixime compared with amoxicillin for treatment of acute otitis media with effusion. Pediatric Infectious Diseases Journal 6: 997–1001, 1987
Nahata MC, Kohibrenner VM, Barson WJ. Pharmacokinetics and cerebrospinal fluid concentrations of cefixime in infants and young children. Chemotherapy 39: 1–5, 1993
Parker SE, Davey PG. Pharmacoeconomics of intravenous drug administration. PharmacoEconomics 1: 103–115, 1992
Patented Medicine Prices Review Board. Fourth Annual Report 1991, pp. 1–63, Ottawa, Canada, 1991
Quintiliani R, Cooper BW, Briceland LL, Nightingale CH. Economic impact of streamlining antibiotic administration. American Journal of Medicine 82 (Suppl. 4A): 391–394, 1987
Reesor NC, Rayani S, Frighetto L, Martinusen S, Mamdani F, et al. Implementation and evaluation of a standardized HS V prophylaxis protocol in a bone marrow transplant program. The Third International Symposium on Oncology Pharmacy Practice (ISOPP III Symposium). The Royal York Hotel, Toronto, Ontario, April 22–24, 1993
Regnier B, et al. A comparative study of IV-ceftriaxone followed by oral cefixime versus parenteral ceftriaxone alone in the treatment of severe upper urinary tract infections. Presse Medicale 18: 1617–1621, 1989
Shalansky S, Gupta S, Jewesson P. Impact of a practical two-stage therapeutic interchange program on aminoglycoside usage. Hospital Formulary 24: 332–341, 1989
Stiver G, Jewesson P, Gupta S. Antibiotic utilization review a necessary evil. Asepsis 9: 2–5, 1987
Vogel F, and the Multicenter Trial Group. Efficacy and tolerance of cefotaxime followed by oral cefixime versus cefotaxime alone in patients with lower respiratory tract infections. Current Therapeutic Research 55 (Suppl. A): 42–48, 1994
Zaremba C, Bachand R, Chow A, Jewesson P. Drug usage review of cefamandole at a major teaching hospital. Canadian Journal of Hospital Pharmacy 41: 195–199, 214, 1988
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jewesson, P. Cost-Effectiveness and Value of an IV Switch. Pharmacoeconomics 5 (Suppl 2), 20–26 (1994). https://doi.org/10.2165/00019053-199400052-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-199400052-00005