Abstract
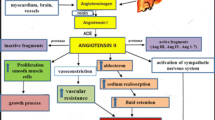
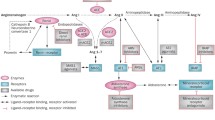
The renin-angiotensin system (RAS) is an ancient and complex cascade of homeostatic reactions aimed at regulating primordial functions that ensure organ perfusion through the control of blood pressure and the regulation of renal-cardiac activity. However, the over-expression or lack of compensatory mechanisms of any of its components may initiate detrimental effects that potentially lead to disease, a balance that makes the RAS a sequence with a labile physiological equilibrium and with a strong harm potential. These characteristics of the RAS in general, and of the angiotensin converting enzyme (ACE) in particular, make it not only an important complex for the regulation of blood pressure and neuropeptide metabolism, but also a fascinating subject of study from a biochemical, evolutionary and genetic point of view.
Pharmacological interventions that influence the RAS by inhibiting the ACE or the angiotensin II type 1 receptor (AT1R) have demonstrated sustained efficacy in reducing the incidence of cardiovascular events and, consequently, vascular mortality in several clinical situations.
ACE inhibitors and angiotensin II receptor antagonists (ARAs) reduce blood pressure and have cardio- and vasculoprotective effects. Anti-atherosclerotic effects have also been attributed to these drugs. For these reasons, it has been hypothesised that RAS inhibitors could also reduce the recurrence of ischaemic events after myocardial revascularisation procedures, namely coronary artery by-pass graft surgery (CABG) or percutaneous coronary interventions (PCI).
Information available on the effect of ACE inhibitors and ARAs in patients with coronary artery disease (CAD) previously treated with revascularisation techniques indicates a substantial reduction of mortality and infarction in these patients. However, data regarding the progression of CAD, restenosis or reocclusion of vascular conduits of the coronary circulation after myocardial revascularisation are inconsistent.
In most studies, the administration of ACE inhibitors neither improved the ischaemic threshold nor reduced the need for new revascularisation procedures. On the contrary, ACE inhibitors have been associated with higher restenosis rates after PCI in some retrospective series. Conversely, a single, exploratory randomised trial demonstrated that the selective AT1R antagonist valsartan significantly reduced stent restenosis after PCI. In patients undergoing CABG, ACE inhibitors did not reduce the risk of graft degeneration or occlusion. Studies that evaluated a possible anti-atherosclerotic effect of ACE inhibitors (including some large randomised trials) have generally been negative.











Similar content being viewed by others
References
Dzau VJ. Mechanism of protective effects of ACE inhibition on coronary artery disease. Eur Heart J 1998; 19 Suppl. J: J2–6
Vanuhotte PM. Endothelial dysfunction and inhibition of converting enzyme. Eur Heart J 1998; 19 Suppl. J: J7–15
Ferrari R. Effect of ACE inhibition on myocardial ischemia. Eur Heart J 1998; 19 Suppl. J: J30–5
Niu T, Chen X, Xu X. Angiotensin converting enzyme gene insertion/deletion polymorphism and cardiovascular disease. Drugs 2002; 62(7): 977–93
Lonn E M, Yusuf S, Jha P, et al. Emerging role of angiotensin-converting enzyme inhibitors in cardiac and vascular protection. Circulation 1994; 90: 2056–69
Dzau VJ, Sasamura H, Hein L. Heterogeneity of angiotensin synthetic pathways and receptor subtypes: physiological and pharmacological implications. J Hypertens 1993; 11 Suppl. 3: S13–22
Urata K, Kinoshita A, Misono K, et al. Identification of a highly specific chymase as the major angiotensin-forming enzyme in the human heart. J Biol Chem 1990; 265: 22348–82
Linz W, Wiemer G, Gohlke P, et al. Contribution of kinins to the cardiovascular actions of angiotensin-converting enzyme inhibitors. Pharmacol Rev 1995; 47: 25–49
Nussberger J, Cugno M, Amstutz C, et al. Plasma bradykinin in angio-oedema. Lancet 1998; 351: 1693–7
Sadoshima J, Izumo S. Molecular characterization of angiotensin II-induced cardiac hypertrophy and hyperplasia of cardiac fibroblasts: critical role of AT1 receptor subtype. Circ Res 1993; 73: 413–23
Schunkert H, Sadoshima J, Cornelius T, et al. Angiotensin-II-induced growth responses in isolated hearts: evidence for load-independent induction of cardiac protein synthesis by angiotensin II. Circ Res 1995; 76: 489–97
Asano K, Dutcher DL, Port JD, et al. Selective downregulation of the angiotensin II AT1 receptor subtype in failing human myocardium. Circulation 1997; 95: 1193–2000
Haywood GA, Gullestad L, Katsuya T, et al. AT1 and AT2 angiotensin receptor gene expression in human heart failure. Circulation 1997; 95: 1201–6
Nakajima M, Hutchinson HG, Fujinaga M, et al. The angiotensin II type 2 (AT2) receptor antagonizes the growth effects of the AT1 receptor: gain-of-function study using gene transfer. Proc Natl Acad Sci U S A 1995; 92: 10663–7
Stoll M, Steckelings U, Paul M, et al. The angiotensin AT2-receptor mediates inhibition of cell proliferation in coronary endothelial cells. J Clin Invest 1995; 95: 651–7
Bartunek J, Weinberg EO, Tajima M, et al. Angiotensin II type 2 receptor blockade amplifies the early signals of cardiac growth response to angiotensin II in hypertrophied hearts. Circulation 1999; 99: 22–5
Burnier M. Angiotensin II type 1 receptor blockers. Circulation 2001; 103: 904–12
The CONSENSUS Trial Study Group. Effect of enalapril on mortality in severe congestive heart failure. N Engl J Med 1987; 316: 1429–35
SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fraction and congestive heart failure. N Engl J Med 1991; 325: 293–302
Garg R, Yusuf S,for the collaborative group on ACE inhibitor trials. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. JAMA 1995; 273: 1450–6
The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1992; 327: 685–91
Pfeffer MA, Braunwald E, Moyè L. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: the SAVE Investigators. N Engl J Med 1992; 327: 669–77
The Acute Ramipril Efficacy (AIRE) Study Investigators. Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. Lancet 1993; 342: 821–8
Torp-Pedersen C, Kober L, for the TRACE study group. Effect of ACE inhibitor trandolapril on life expectancy of patients with reduced left-ventricular function after myocardial infarction. Lancet 1999; 354: 9–12
Pitt B, O’Neill B, Feldman R, et al. The Quinalapril Ischemic Event Trial (QUIET): evaluation of chronic ACE inhibitor therapy in patients with ischemic heart disease and preserved left ventricular function. Am J Cardiol 2001; 87: 1058–63
Yusuf S, Sleiht P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients: the Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 2000; 342: 145–53
Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus; results of the HOPE study and MICRO-HOPE substudy. Lancet 2000; 355: 253–9
Francis GS. ACE inhibition in cardiovascular disease. N Engl J Med 2000; 342: 201–2
The European trial on Reduction Of cardiac events with Per-indopril on stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo controlled, multicentre trial (the EUROPA study). Lancet 2003; 362: 782–7
Lee VC, Rhew DC, Dylan M, et al. Meta-analysis: angiotensin-receptor blockers in chronic heart failure and high-risk acute myocardial infarction. Ann Intern Med 2004; 141: 693–704
Dahlof B, Devereux R, Kjieldsen S, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359: 995–1003
Julius S, Kjieldsen SE, Brunner H, et al. VALUE trial: long-term blood pressure trends in 13,449 patients with hypertension and high cardiovascular risk. Am J Hyper 2003; 16: 544–8
Pitt B, Poole-Wilson PA, Segal R, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial. The Losartan Heart Failure Survival Study ELITE II. Lancet 2000; 355: 1582–7
Cohn JN, Tognoni G, for the Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345: 1667–75
Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM Preserved Trial. Lancet 2003; 362: 777–81
Granger C, McMurray J, Held P, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting enzyme inhibitors: the CHARM-Alternative trial. Lancet 2003; 362: 772–6
McMurray J, Ostergren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting enzyme inhibitors: the CHARM-Added trial. Lancet 2003; 362: 767–71
Pfeffer MA, McMurray JJV, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both: the Valsartan in Acute Myocardial Infarction Trial Investigators. N Engl J Med 2003; 349: 1893–906
Dickstein K, Kjekshus J, OPTIMAAL Steering committee of the OPTIMAAL Study Group. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Optimal trial in myocardial infarction with angiotensin antagonist losartan. Lancet 2002; 360: 752–60
Keane WF, Brenner BM, de Zeeuw D, et al. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int 2003; 63: 1499–507
Ohishi M, Ueda M, Rakugi H, et al. Upregulation of angiotensin-converting enzyme during the healing process after injury at the site of percutaneous transluminal coronary angioplasty in humans. Circulation 1997; 96: 3328–37
Schieffer B, Schieffer E, Hilfiker-Kleiner D, et al. Expression of angiotensin II and interleukin 6 in human coronary atherosclerotic plaques: potential implications for inflammation and plaque instability. Circulation 2000; 101: 1372–8
Radomski MW, Palmer RMJ, Moncada S, et al. L-arginine-nitric oxide pathway present in human platelets regulates aggregation. Proc Natl Acad Sci U S A 1990; 87: 5193–7
Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A 1991; 88: 4651–5
Bonithon-Kopp C, Ducimetiere P, Touboul PJ, et al. Plasma angiotensin-converting enzyme activity and carotid wall thickening. Circulation 1994; 89: 952–4
Ribichini F, Steffenino G, Dellavalle A, et al. Plasma activity and insertion/deletion polymorphism of angiotensin I-converting enzyme: a major risk factor and a marker of risk for coronary stent restenosis. Circulation 1998; 97: 147–54
Rakugi H, Kim DK, Krieger JE, et al. Induction of angiotensin converting enzyme in the neointima after vascular injury: possible role in restenosis. J Clin Invest 1994; 93: 339–46
Powell JS, Clozel JP, Muller RKM, et al. Inhibitors of angiotensin-converting enzyme prevent myointimal proliferation after vascular injury. Science 1989; 245: 186–8
Lonn E, Yusuf S, Dzavik V, et al. Effects of ramipril and vitamin E on atherosclerosis: the study to evaluate carotid ultrasound changes in patients treated with ramipril and vitamin E (SECURE). Circulation 2001; 103: 919–25
Oosterga M, Voors A, Pinto Y, et al. Effects of quinalapril on clinical outcomes after coronary artery by-pass grafting (the QUO VADIS study). Am J Cardiol 2001; 87: 542–6
Kjoller-Hansen L, Steffensen R, Grande P. The Angiotensin-converting enzyme inhibition Post REvascularization Study (APRES). J Am Coll Cardiol 2000; 35: 991–8
Cashin-Hemphill L, Holmvang G, Chan RC, et al. Angiotensin-converting enzyme inhibition as antiatherosclerotic therapy: no answer yet. The QUIET Investigators. Am J Cardiol 1999; 83: 43–7
Pepine CJ, Rouleau JL, Annis K, et al. Effects of angiotensin-converting enzyme inhibition on transient ischemia: the Quinalapril Anti-ischemia and Symptoms of Angina Reduction (QUASAR) trial. J Am Coll Cardiol 2003; 42: 2049–59
MacMahon S, Sharpe N, Gamble G, et al. Randomised, placebo-controlled trial of the angiotensin-converting enzyme inhibitor, ramipril, in patients with coronary or other occlusive arterial disease: the PART-2 Collaborative Research Group. J Am Coll Cardiol 2000; 36: 438–43
Teo KK, Burton JR, Buller CE, et al. Long-term effects of cholesterol lowering and angiotensin-converting enzyme inhibition on coronary atherosclerosis: the Simvastatin/Enalapril Coronary Atherosclerosis Trial (SCAT). The SCAT Investigators. Circulation 2000; 102: 1748–54
Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20536 high-risk individuals: a randomized placebo-controlled trial. Lancet 2002; 360: 7–22
Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350: 1495–504
Serruys PW, de Feyter P, Macaya C, et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 287: 3215–22
Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 2004; 291: 1071–80
Borland JA, Chester AH, Crabbe S, et al. Differential action of angiotensin II and activity of angiotensin-converting enzyme in human bypass graft. J Thorac Cardiovasc Surg 1998; 116: 206–12
Yang Z, Oemar BS, Carrel T, et al. Different proliferative properties of smooth muscle cells of human arterial and venous bypass vessels. Circulation 1998; 97: 181–7
The Writing Committee for the Prevention of Events with Angiotensin Converting Enzyme inhibition (PEACE) Trial. Angiotensin-converting-enzyme inhibition in stable coronary artery disease: the PEACE Trial Investigators. N Engl J Med 2004; 351: 2058–68
Massie BM, Teerlink JR. Interaction between aspirin and angiotensin-converting enzyme inhibitors: real or imagined. Am J Med 2000; 109: 431–3
Cleland JGF, Hendersson E, McLenachan J, et al. Effects of captopril, an angiotensin-converting enzyme inhibitor in patients with angina pectoris and heart failure. J Am Coll Cardiol 1991; 17: 733–9
Gibbs JSR, Crean PA, Mockus P, et al. The variable effects of angiotensin converting enzyme inhibition on myocardial ischaemia in chronic stable angina. Br Heart J 1989; 62: 112–7
Steffensen R, Grande P, Kyst Madsen J, et al. Short-term effects of captopril on exercise tolerance in patients with chronic stable angina pectoris and normal left ventricular function. Cardiology 1995; 86: 445–50
Vogt M, Motz W, Strauer BE. ACE-inhibitors in coronary artery disease? Basic Res Cardiol 1993; 88 Suppl. 1: 43–6
Nguyen KN, Aursen I, Kjekshus J. Interaction between enalapril and aspirin on mortality after acute myocardial infarction: subgroup analysis of the Cooperative New Scandinavian Enalapril Survival Study II (CONSENSUS II). Am J Cardiol 1997; 79: 115–9
Al-Khadra AS, Salem DN, Rand WM, et al. Antiplatelet agents and survival: a cohort analysis from the Studies Of Left Ventricular Dysfunction (SOLVD) trial. J Am Coll Cardiol 1998; 31: 419–25
Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature 1971; 231: 232–5
Hall D, Zeitler H, Rudolph W. Counteraction of the vasodilator effects of enalapril by aspirin in severe heart failure. J Am Coll Cardiol 1992; 20: 1549–55
Spaulding C, Charbonnie B, Cohen-Solal A. Acute hemodynamic interaction of aspirin and ticlopidine with enalapril. Circulation 1998; 98: 757–65
Leor J, Reicher-Reiss H, Goldbourt U, et al. Aspirin and mortality in patients treated with angiotensin-converting enzyme inhibitors. J Am Coll Cardiol 1999; 33: 1920–5
Teo KK, Yusuf S, Pfeffer M, et al. Effects of long-term treatment with angiotensin-converting-enzyme inhibitors in the presence or absence of aspirin: a systematic review. Lancet 2002; 360: 1037–43
Multicenter European Research trial with Cilazapril after Angioplasty to prevent Transluminal coronary Obstruction and Restenosis (MERCATOR) Study Group. Does the new angiotensin converting enzyme inhibitor cilazapril prevent restenosis after percutaneous transluminal coronary angioplasty? Results of the MERCATOR study: a multicenter, randomised, double blind placebo-controlled trial. Circulation 1992; 86: 100–10
Faxon DP,the Multicenter American Research trial with Cilazapril after Angioplasty to Prevent Transluminal Coronary Obstruction and Restenosis (MARCATOR) Study Group. Effect of high dose angiotensin-converting enzyme inhibition on restenosis: final results of the MARCATOR study, a multicenter, double-blind, placebo-controlled trial of cilazapril. J Am Coll Cardiol 1995; 25: 362–9
Prat RE, Dzau VJ. Pharmacological strategies to prevent restenosis: lessons learned from blockade of the renin-angiotensin system. Circulation 1996; 93: 848–52
Dzau VJ, Re R. Tissue angiotensin system in cardiovascular medicine: a paradigm shift. Circulation 1994; 89: 493–8
Currier JW, Faxon DP. Restenosis after percutaneous transluminal coronary angioplasty: have we been aiming at the wrong target? J Am Coll Cardiol 1995; 25: 516–20
Faxon DP. Systemic drug therapy for restenosis: ‘deja vu all over again’. Circulation 2002; 106: 2296–8
Mintz GS, Popma JJ, Hong MK, et al. Intravascular ultrasound to discern device-specific effects and mechanism of restenosis. Am J Cardiol 1996; 78 Suppl. 3A: 18–22
Hoffmann R, Mintz GS, Dussaillant GR, et al. Patterns and mechanism of in-stent restenosis. a serial intravascular ultrasound study. Circulation 1996; 94: 1247–54
Mintz GS, Popma JJ, Pichard A, et al. Arterial remodeling after coronary angioplasty: a serial intravascular ultrasound study. Circulation 1996; 94: 35–43
Painter JA, Mintz GS, Wong SC, et al. Serial intravascular ultrasound studies fail to show evidence of chronic Palmaz Schatz stent recoil. Am J Cardiol 1995; 75: 398–400
Farb A, Sangiorgi G, Carter A, et al. Pathology of acute and chronic coronary stenting in humans. Circulation 1999; 99: 44–52
Farb A, Weber DK, Kolodgie FD, et al. Morphological predictors of restenosis after coronary stenting in humans. Circulation 2002; 105: 2974–80
Farb A, Burke AP, Kolodgie FD, et al. Pathological mechanisms of fatal late coronary stent thrombosis in humans. Circulation 2003; 108: 1701–6
Ribichini F. Current perspective: in-stent restenosis. Ital Heart J 2001: 2; 728–735
Ferrero V, Ribichini F, Matullo G, et al. Estrogen Receptor alpha polymorphisms and angiographic outcome after coronary artery stenting. Arterioscler Thromb Vasc Biol 2003; 23: 2223–8
Ribichini F, Pugno F, Ferrero V, et al. Angiotensin-converting enzyme tissue activity in the diffuse in-stent restenotic plaque. Circulation 2000; 101: e33–5
Ribichini F, Matullo G, Piazza A, et al. Relationship between plasma ACE activity and the proliferative healing process in coronary vessel injury after coronary stenting. Atherosclerosis 2000; 152: 261–3
Ribichini F, Ferrero V, Matullo G, et al. Association study of the i/d polymorphism and plasma angiotensin-converting enzyme (ACE) as risk factors for stent restenosis. Clin Sci 2004; 107(4): 381–9
Hahn AW, Jonas U, Buhler FR, et al. Activation of human peripheral monocytes by angiotensin II. FEBS Lett 1994; 347: 178–80
Naftilan AJ, Ryan TJ, Pratt RE, et al. Location and differential regulation of angiotensinogen mRNA expression in the vessel wall. J Clin Invest 1991; 87: 1300–11
Rakugi H, Jacob HJ, Krieger JE, et al. Vascular injury induces angiotensinogen gene expression in the media and the neointima. Circulation 1993; 87: 283–90
Ribichini F, Pugno F, Ferrero V, et al. Histologie and immunocytochemical analysis of de novo coronary atherosclerotic plaques of diabetic and non-diabetic patients [abstract]. Circulation 2000; 102: 3117
Ribichini F, Pugno F, Ferrero V, et al. Angiotensin-converting enzyme immunoreactivity is associated with macrophage-infiltration and cell proliferation in ‘de novo’ coronary plaques [abstract]. Eur Heart J 2002; 23: P1230
Depres C, Ribichini F, Wijns W. Pathophysiological insights from studies on retrieved tissue. Semin Interv Cardiol 2000; 5: 175–84
Hoshida S, Kato J, Nishino M, et al. Increased angiotensin-converting enzyme activity in coronary artery specimens from patients with acute coronary syndrome. Circulation 2001; 103: 630–3
Diet F, Pratt RE, Berry GJ, et al. Increased accumulation of tissue ACE in human atherosclerotic coronary artery disease. Circulation 1996; 94: 2756–69
Yu CM, Tipoe GL, Wing-Hon La K, et al. Effects of combination of angiotensin-converting enzyme inhibitor and angiotensin receptor antagonist on inflammatory cellular infiltration and myocardial interstitial fibrosis after acute myocardial infarction. J Am Coll Cardiol 2001; 38: 1207–15
Soejima H, Ogawa H, Yasue H, et al. Angiotensin-converting enzyme inhibition reduces monocyte chemoattractant protein-1 and tissue factor levels in patients with myocardial infarction. J Am Coll Cardiol 1999; 34: 983–8
Neri Serneri GG, Boddi M, Modesti PA, et al. Cardiac angiotensin II participates in coronary microvessel inflammation of unstable angina and strengthens the immunomediated component. Circ Res 2004; 94: 1630–7
Laporte S, Perodin J, Bourgeoise R, et al. What can angiotensin antagonists do that converting-enzyme inhibition can’t: the case of post-angioplastic restenosis. Can J Physiol Pharmacol 1996; 74: 867–77
O’Keefe JH, Wetzel M, Moe RR, et al. Should an angiotensin-converting enzyme inhibitor be standard therapy for patients with atherosclerotic disease? J Am Coll Cardiol 2001; 37: 1–8
Wilensky RL. Angiotensin-receptor blockers: revival of the systemic prevention of restenosis? Cardiovasc Drug Ther 2003; 17: 63–73
Meurice T, Bauters C, Hermant X, et al. Effect of ACE inhibitors on angiographic restenosis after coronary stenting (PARIS): a randomised, double-blind, placebo-controlled trial. Lancet 2001; 357: 1321–4
Rigat B, Hubert C, Alhenc-Gelas F, et al. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest 1990; 86: 1343–6
Cambien F, Poirier O, Lecerf L, et al. Deletion polymorphism in the gene for angiotensin-converting enzyme is a potent risk factor for myocardial infarction. Nature 1992; 359: 641–4
Raynolds MV, Bristow MR, Bush EW, et al. Angiotensin-converting enzyme DD genotype in patients with ischaemic or idiopathic dilated cardiomyopathy. Lancet 1993; 342: 1073–5
Montgomery HE, Clarkson P, Dollery CM, et al. Association of angiotensin-converting enzyme gene I/D polymorphism with change in left ventricular mass in response to physical training. Circulation 1997; 96: 741–7
Okumura K, Sone T, Kondo J, et al. Quinalapril prevents restenosis after coronary stenting in patients with angiotensin-converting enzyme D allele. Circ J 2002; 66: 311–6
Kondo J, Sone T, Tsuboi H, et al. Effect of quinapril on intimai hyperplasia after coronary stenting as assessed by intravascular ultrasound. Am J Cardiol 2001; 87: 443–5
Toyofyuku M, Imazu K, Yamamoto H, et al. Influence of angiotensinogen M253T gene polymorphism and an angiotensin converting enzyme inhibitor on restenosis after percutaneous coronary intervention. Atherosclerosis 2002; 160: 339–44
Okimoto T, Imazu M, Hayashi Y, et al. Quinalapril with high affinity to tissue angiotensin-converting enzyme reduces restenosis after percutaneous transcatheter coronary intervention. Cardiovasc Drugs Ther 2001; 15: 323–9
Jorgensen E, Kelbaek H, Helqvist S, et al. Predictors of coronary in-stent restenosis: importance of angiotensin-converting enzyme gene polymorphism and treatment with angiotensin-converting enzyme inhibitors. J Am Coll Cardiol 2001; 38: 1434–9
Ribichini F, Wijns W, Ferrero V, et al. Effect of angiotensin-converting enzyme inhibition on restenosis after coronary stenting. Am J Cardiol 2003; 91: 154–8
Koch W, Mehilli J, von Beckerath N, et al. Angiotensin I-converting enzyme inhibitors and restenosis after coronary artery stenting in patients with the DD genotype of the ACE gene. J Am Coll Cardiol 2003; 41: 1957–61
Ellis SG, Lincoff AM, Whitlow PL, et al. Evidence that angiotensin-converting enzyme inhibitor use diminishes the need for coronary revascularization after stenting. Am J Cardiol 2002; 89: 937–40
Kastrati A, Mehilli J, Dirschinger J, et al.Restenosis after coronary placement of various stent types. Am J Cardiol 2001; 87: 34–9
Miyauchi K, Kawai S, Okada R, et al. Limitations of angiotensin-converting enzyme inhibitor in restenosis of a deep arterial injury model. Circ J 1998; 62: 53–60
Kalinowski M, Tepe G, Schieber A, et al. Local administration of ramiprilat is less effective than oral ramipril in preventing restenosis after balloon angioplasty in an animal model. J Vasc Interv Radiol 1999; 10(10): 1397–404
Li J, Wanchun C. Benazepril on tissue angiotensin-converting enzyme and cellular proliferation in restenosis after experimental angioplasty. J Cardiovasc Pharm 1997; 30: 790–7
Huckle WR, Drag MD, Acker WR, et al. Effects of subtype-selective and balanced angiotensin II receptor antagonists in a porcine coronary artery model of vascular restenosis. Circulation 1996; 93: 1009–19
Virmani R, Kolodgie F, Farb A, et al. Drug eluting stents: are human and animal studies comparable? Heart 2003; 89: 133–8
Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med 2002; 346: 1773–80
Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in native coronary arteries: the SIRIUS Investigators. N Engl J Med 2003; 349: 1315–23
Schofer J, Schlüter M, Gershilck AH, et al. Sirolimus-eluting stents for the treatment of patients with long atherosclerotic lesions in small coronary arteries: a double-blind, randomised controlled trial (E-SIRIUS). The E-SIRIUS Investigators. Lancet 2003; 362: 1093–9
Stone GW, Ellis SG, Cox DA, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease: the TAXUS-IV Investigators. N Engl J Med 2004; 350: 221–31
Schampaert E, Cohen EA, Schlüter M, et al. The Canadian study of the sirolimus-eluting stent in the treatment of patients with long de-novo lesions in small native coronary arteries (C-SIRIUS). J Am Coll Cardiol 2004; 43: 1110–5
Abizaid A, Albertal M, Costa MA, et al. First human experience with the 17-beta-estradio-eluting stent: the Estrogen And Stents To Eliminate Restenosis (EASTER) trial. J Am Coll Cardiol 2004; 43: 1118–21
Van Beusekom HMM, Ferrero V, Harteveld MS, et al. Quinaprilat coated stents do not attenuate intimai thickening in a porcine coronary model [abstract]. Eur Heart J 2003; 23: 3575
Peters S, Gotting B, Trummel M, et al. Valsartan for prevention of restenosis after stenting of type B2/C lesions: the VAL-PREST trial. J Invasive Cardiol 2001; 13: 93–7
Zhuo JL, Mendelsohn FA, Ohishi M. Perindopril alters vascular angiotensin-converting enzyme, AT(1) receptor, and nitric oxide synthase expression in patients with coronary heart disease. Hypertension 2002; 39: 634–8
Ribichini F, Ferrero V, Wijns W, et al. Can ACE inhibitors promote detrimental vascular effects after percutaneous injury? Hypertension 2002; 40: e5–6
Hall JM. Bradykinin receptors: pharmacological properties and biological roles. Pharmacol Ther 1992; 56: 131–90
Braun A, Kammerer S, Maier E, et al. Polymorphisms in the gene for the human B2-bradykinin receptor: new tools in assessing a genetic risk for bradykinin-associated diseases. Immunopharmacology 1996; 33: 32–5
Dhamrait SS, Payne JR, Li P, et al. Variation in bradykinin receptor genes increases the cardiovascular risk associated with hypertension. Eur Heart J 2003; 24: 1672–80
Hallberg P, Lind L, Michaelsson K, et al. B2 bradykinin (B2BKR) polymorphism and change in left ventricular mass in response to antihypertensive treatment: results from the Swedish Irbesartan Left Ventricular Hypertrophy Investigation versus Atenolol (SILVHIA) trial. J Hypertens 2003; 21: 621–4
Acknowledgements
The authors would like to thank Professor Gianni Bussolati, from the Dipartimento di Scienze Biomediche, Università di Torino, Torino, Italy, for his valuable contribution in performing the immunochemical studies.
The authors have no conflict of interest regarding the opinions expressed in this manuscript and did not receive grants or financial support from industry or from any other source to prepare this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ribichini, F., Ferrero, V., Rognoni, A. et al. Angiotensin Antagonism in Coronary Artery Disease. Drugs 65, 1073–1096 (2005). https://doi.org/10.2165/00003495-200565080-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200565080-00004