Abstract
-
▴ Fludarabine is an antimetabolite antineoplastic agent used in the treatment of various haematological malignancies, particularly B-cell chronic lymphocytic leukaemia (CLL).
-
▴ An oral formulation of fludarabine has recently become available in the majority of European countries for the treatment of patients with relapsed or refractory B-cell CLL after initial treatment with an alkylating agent-based regimen. It is the first oral formulation of a purine analogue available for clinical use in B-cell CLL.
-
▴ Pharmacokinetic studies evaluating the bioavailability of oral fludarabine indicate that an oral dose of 40 mg/m2/day would provide similar systemic drug exposure to the standard intravenous dose of 25 mg/m2/day.
-
▴ A phase II study evaluated the clinical efficacy of six to eight cycles of oral fludarabine 40 mg/m2/day for 5 days of each 28-day cycle in 78 patients with previously treated B-cell CLL. Depending on the criteria used, the overall response rate was 46.2% (20.5% complete response [CR], 25.6% partial response [PR]) or 51.3% (17.9% CR, 33.3% PR). These results were similar to the 48% overall response rate reported in a similar historical control group treated with intravenous fludarabine.
-
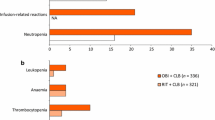
▴ Myelosuppression (WHO grade 3 or 4) was the most frequently reported adverse effect with oral fludarabine therapy. Other common adverse effects included infection and gastrointestinal disturbances, although these were usually of mild to moderate severity (WHO grade 1 or 2). Overall, the tolerability profile of oral fludarabine is similar to that of the intravenous formulation.


Similar content being viewed by others
Notes
Use of tradenames is for identification purposes only and does not imply product endorsement.
References
Adkins JC, Peters DH, Markham A. Fludarabine: an update of its pharmacology and use in the treatment of haematological malignancies. Drugs 1997 Jun; 53(6): 1005–37
National Cancer Institute. Chronic lymphocytic leukemia (PDQ®): treatment [online]. Available from URL: http://www.cancer.gov/cancer_information/ [Accessed 2003 Jul 15]
National Institute for Clinical Excellence. Technology Appraisal Guidance. No. 29. Guidance on the use of fludarabine for B-cell chronic lymphocytic leukaemia. London: National Institute for Clinical Excellence, 2001 Sep: 14
Schering AG. Fludara® (fludarabine phosphate) receives marketing approval in the UK as first-line treatment for B-cell chronic lymphocytic leukemia (media release 2003 Feb 11) [online]. Available from URL: http://www.schering.co.uk [Accessed 2003 Jun 12]
Kalil N, Cheson BD. Management of chronic lymphocytic leukaemia. Drugs Aging 2000 Jan; 16: 9–27
Kay NE, Hamblin TJ, Jelinek DF, et al. Chronic lymphocytic leukemia. Hematology (Am Soc Hematol Educ Program) 2002, 193-213
Montserrat E. Current and developing chemotherapy for CLL. Med Oncol 2002; 19 Suppl.: S11–9
Hamblin TJ. Achieving optimal outcomes in chronic lymphocytic leukaemia. Drugs 2001; 61(5): 593–611
Keating MJ. Chronic lymphocytic leukemia. Semin Oncol 1999; 26 Suppl. 14(5): 107–14
Schering AG. Schering launches oral formulation of Fludara® (media release 2003 Jan 22) [online]. Available from URL: http://www.schering.co.uk [Accessed 2003 Jun 9]
British National Formulary. 45th edition ed. London: British Medical Association and Royal Pharmaceutical Society of Great Britain, 2003
Gandhi V, Plunkett W. Cellular and clinical pharmacology of fludarabine. Clin Pharmacokinet 2002; 41(2): 93–103
O’Rourke TJ, Burris HA, Rodriguez GI, et al. Phase I pharmacokinetic and bioavailability study of five daily intravenous and oral doses of fludarabine phosphate in patients with advanced cancer [abstract no. 736]. 33rd Annual Meeting of the American Society of Clinical Oncology; 1997 May 17–20; Denver, 210a
Kemena A, Keating M, Plunkett W. Plasma and cellular bioavailability of oral fludarabine [abstract no. 199]. Blood 1991 Nov 15; Suppl. 1(10): 52a
Kemena A, Keating M, Plunkett W. Oral bioavailability of plasma fludarabine and fludarabine triphosphate (F-ara-ATP) in peripheral CLL cells [abstract no. 238]. Onkologie 1991 Oct; 14 (Suppl. 2): 83
Oscier D, Orchard JA, Culligan D, et al. The bioavailability of oral fludarabine phosphate is unaffected by food. Hematol J 2001; 2(5): 316–21
Foran JM, Oscier D, Orchard J, et al. Pharmacokinetic study of single doses of oral fludarabine phosphate in patients with “low-grade” non-Hodgkin’s lymphoma and B-cell chronic lymphocytic leukemia. J Clin Oncol 1999 May; 17(5): 1574–9
Klein M, Ludwig W-D, Fülle HH, et al. 2F-ara-A pharmacokinetics including systemic availability during treatment with fludarabine phosphate given either perorally as an immediate release tablet formulation or intravenously as a solution [abstract no. 525]. Ontologie 1997 Oct; 20 Suppl. 1: 137
Boogaerts MA, Van Hoof A, Catovsky D, et al. Activity of oral fludarabine phosphate in previously treated chronic lymphocytic leukemia. J Clin Oncol 2001 Nov 15; 19(22): 4252–8
Johnson S, Smith AG, Löffler H, et al. Multicentre prospective randomised trial of fludarabine versus cyclophosphamide, doxorubicin, and prednisone (CAP) for treatment of advancedstage chronic lymphocytic leukaemia. The French Cooperative Group on CLL. Lancet 1996; 347: 1432–8
Rossi J-F, Van Hoof A, De Bock R, et al. Oral fludarabine phosphate as first-line treatment of chronic lymphocytic leukemia [abstract no. 1491 plus poster no. 657-1]. Blood 2002 Nov 16; 100(11 Pt 1): 384–5
Cazin B, Maloum K, Divine M, et al. Oral fludarabine and cyclophosphamide in previously untreated CLL: preliminary data on 75 pts [abstract no. 773]. Blood 2002 Nov 16; 100 (11 Pt 1): 206a
Mosby’s Drug Consult: Fludarabine phosphate [online]. Available from URL: http://www.mosbydrugs.com [Accessed 2003 Jul 7]
Cid J, Revilla M, Cervera A, et al. Progressive multifocal leukoencephalopathy following oral fludarabine treatment of chronic lymphocytic leukemia. Ann Hematol 2000 Jul; 79(7): 392–5
Cohen RB, Abdallah JM, Gray JR, et al. Reversible neurologic toxicity in patients treated with standard-dose fludarabine phosphate for mycosis fungoides and chronic lymphocytic leukemia. Ann Intern Med 1993; 118: 114–6
Johnson PWM, Fearnley J, Domizio P, et al. Neurological illness following treatment with fludarabine. Br J Cancer 1994; 70: 966–8
Zabernigg A, Maier H, Thaler J, et al. Late-onset fatal neurological toxicity of fludarabine [letter]. Lancet 1994; 344: 1780
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Plosker, G.L., Figgitt, D.P. Oral Fludarabine. Drugs 63, 2317–2323 (2003). https://doi.org/10.2165/00003495-200363210-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200363210-00004