Summary
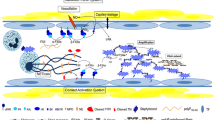
Bacterial products [lipopolysaccharide (LPS) with Gram-negative bacteria and toxins, superantigens or cell wall fragments with Gram-positive bacteria] are the main activators of the septic shock cascade. These molecules interact with monocytes, macrophages and endothelial cells to produce inflammatory cytokines [tumour necrosis factor (TNF) and interleukins 1 and 6], and may activate other harmful pathways such as the coagulation system, complement cascade and lipid mediators.
As a therapeutic strategy, antibodies directed against LPS have been well studied, although, on the whole, the clinical results have been disappointing. Other possible interventions that have not yet been tested clinically include natural intracellular antibacterial proteins (e.g. bacterial permeability-increasing protein) and high density lipoprotein (responsible for detoxifying LPS in the body).
The stimulation pathway of responsive cells by bacterial products is also another possible target for intervention. Compounds under investigation include soluble CD14 and antibodies directed against CD14 or LPS binding protein. Antibodies directed against the cytokines are another option. Anti-TNF antibodies are currently being investigated, but conclusive evidence of their activity is still lacking. Soluble receptors (e.g. interleukin-1 receptor antagonist, or soluble TNF receptor) are another possibility; one soluble TNF receptor is still undergoing clinical investigation.
Similar content being viewed by others
References
Anon. Increase in national hospital discharge survey rates for septicemia — United States, 1979–1987. MMWR 1990; 39: 31–4.
Bone RC. Gram-positive organisms and sepsis. Arch Intern Med 1994; 154: 26–34.
Weidemann B, Brade H, Rietschel ET, et al. Soluble peptidoglycan-induced monokine production can be blocked by anti-CD14 monoclonal antibodies and by lipid A partial structures. Infect Immun 1994; 62: 4709–15.
Heumann D, Gallay P, Barras C, et al. Control of lipopolysaccharide (LPS) binding and LPS-induced tumor necrosis factor secretion in human peripheral blood monocytes. J Immunol 1992; 148: 3505–12.
Wright SD, Ramos RA, Tobias PS, et al. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990; 249: 1431–3.
Ulevitch RJ, Tobias PS. Recognition of endotoxin by cells leading to transmembrane signalling. Curr Opin Immunol 1994; 6: 125–30.
Heumann D, Barras C, Severin A, et al. Gram-positive cell walls stimulate synthesis of tumour necrosis factor alpha and interleukin-6 by human monocytes. Infect Immun 1994; 62: 2715–21.
Ziegler EJ, Fisher CJ, Sprung CL, et al. Treatment of Gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin: a randomised, double-blind, placebo-controlled trial. New Engl J Med 1991; 324: 429–36.
Anon. Another sepsis drug down: Chiron’s T-88 shows no patient survival benefit. Biotechnol Newsl 1994; May 16: 4
Greenman RL, Schein RMH, Martin MA, et al. A controlled clinical trial of E5 murine monoclonal IgM antibody to endotoxin in the treatment of Gram-negative sepsis. JAMA 1991; 266: 1097–1102.
Bone RC, Balk RA, Fein AM, et al. A second large controlled clinical study of E5, a monoclonal antibody to endotoxin: results of a prospective, multicenter, randomized, controlled trial. Crit Care Med 1995; 23: 994–1005.
Di Padova F, Brade H, Barclay GR, et al. A broadly cross-protective monoclonal antibody binding to Escherichia coli and Salmonella lipopolysaccharides. Infect Immun 1993; 61: 3863–72.
Wilde CG, Seilhamer JJ, McGrogan M, et al. Bactericidal/permeability-increasing protein and lipopolysaccharide (LPS)-binding protein: LPS binding properties and effects on LPS-mediated cell activation. J Biol Chem 1994; 269: 17411–6.
Levine DM, Parker TS, Donnelly TM, et al. In vivo protection against endotoxin by plasma high density lipoprotein. Proc Natl Acad Sci USA 1993; 90: 12040–4.
Haziot A, Rong G-W, Bazil V, et al. Recombinant soluble CD14 inhibits LPS-induced tumor necrosis factor-α production by cells in whole blood. J Immunol 1994; 152: 5868–76.
Haziot A, Rong GW, Lin XY, et al. Recombinant soluble CD14 prevents mortality in mice treated with endotoxin (lipopolysaccharide). J Immunol 1995; 154: 6529–32.
Leturcq D, Moriarty AM, Talbott G, et al. Therapeutic strategies to block LPS interactions with its receptor. In: Levin J, Alving CR, Munford RS, et al. editors. Bacterial endotoxins: lipopolysaccharides from genes to therapy. New York: Wiley-Liss Inc, 1995: 473–7.
Gallay P, Heumann D, Le Roy D, et al. Mode of action of anti-lipopolysaccharide-binding protein antibodies for prevention of endotoxemic shock in mice. Proc Natl Acad Sci USA 1994; 91: 7922–6.
Gallay P, Heumann D, Le Roy D, et al. Lipopolysaccharidebinding protein as a major plasma protein responsible for endotoxemic shock. Proc Natl Acad Sci USA 1993; 90: 9935–8.
Tracey KJ, Fong Y, Hesse DG, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 1987; 330: 662–4.
Fisher CJ, Opal SM, Dhainaut JF, et al. Influence of an anti-tumor necrosis factor monoclonal antibody on cytokine levels in patients with sepsis. Crit Care Med 1993; 21: 318–27.
Reinhart K, Weigand-Löhnert C, Grimmer F, et al. Safety and efficacy of the monoclonal anti-TNF fragment MAK 195F in patients with sepsis and septic shock [abstract]. Shock 1995; 3 (Suppl.): 63.
Wherry J, Wenzel R, Wunderink R, et al. Monoclonal antibody to human necrosis factor (TNF MAb): multi-center efficacy and safety study in patients with the sepsis syndrome [abstract no. 696]. Proceedings of the 33rd Interscience Conference on Antimicrobial Agents and Chemotherapy; 1993 Oct 18: New Orleans: 1993
Abraham E, Wunderink R, Silverman H, et al. Efficacy and safety of monoclonal antibody to human tumor necrosis factor a in patients with sepsis syndrome: a randomized, controlled, double-blind, multicenter clinical trial. JAMA 1995; 273: 934–41.
Carlet J, Cohen J, Andersson J, et al. An international efficacy and safety study of monoclonal antibody to human necrosis factor in patients with the sepsis syndrome [abstract no. B/1]. Proceedings of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1994 Oct 4–7: Orlando: 1994
Mancilla J, Garcia P, Dinarello CA. The interleukin-receptor antagonist can either reduce or enhance the lethality of Klebsiella pneumoniae sepsis in newborn rats. Infect Immun 1993; 61: 926–32.
Wakabayashi G, Gelfand JA, Burke JF, et al. A specific antagonist for interleukin 1 prevents Escherichia coli-induced shock in rabbits. FASEB J 1991; 5: 338–43.
Alexander HR, Doherty GM, Buresh CM, et al. A recombinant human receptor antagonist to interleukin 1 improves survival after lethal endotoxemia in mice. J Exp Med 1991; 173: 1029–32.
Fisher Jr CJ, Slotman GJ, Opal SM, et al. Initial evaluation of human recombinant interleukin 1 receptor antagonist in the treatment of sepsis syndrome: a randomized, open-label, placebo-controlled trial. Crit Care Med 1994; 22: 12–21.
Fischer Jr CJ, Dhainaut J-FA, Opal SM, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome: results from a randomized, double-blind, placebo-controlled trial. JAMA 1994; 271: 1836–43.
Anon. Synergen drops Antril or sepsis. Scrip 1994; 1942: 10.
Dembic Z, Loetscher H, Gubler U, et al. Two human TNF receptors have similar extracellular, but distinct intracellular, domain sequences. Cytokine 1990; 2: 231–7.
Loetscher H, Angehrn P, Schlaeger EJ, et al. Efficacy of a chimeric TNFR-IgG fusion protein to inhibit TNF activity in animal models of septic shock. In: Levin J, Alving CR, Munford RS, et al., editors. Bacterial endotoxin: recognition and effector mechanisms. Amsterdam: Elsevier Science Publishers BV, 1993: 455–62.
Mohler KM, Torrance DS, Smith CA, et al. Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. J Immunol 1993; 151: 1548–61.
Agosti JM, Fisher CJ, Opal SM, et al. Treatment of sepsis syndrome with soluble TNF receptor (sTNFR) [abstract no. M4]. Proceedings of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1994 Oct 4–7: Orlando: 1994; 65
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Glauser, M.P. The Inflammatory Cytokines. Drugs 52 (Suppl 2), 9–17 (1996). https://doi.org/10.2165/00003495-199600522-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199600522-00004