Summary
Synopsis
Torasemide (torsemide) is a high-ceiling loop diuretic which acts on the thick ascending limb of the loop of Henle to promote rapid and marked excretion of water, sodium and chloride. Like furosemide (frusemide), its major site of action is from the luminal side of the cell. Torasemide is at least twice as potent as furosemide on a weight-for-weight basis, produces equivalent diuresis and natriuresis at lower urinary concentrations and has a longer duration of action, allowing once-daily administration without the paradoxical antidiuresis seen with furosemide. Torasemide also appears to promote excretion of potassium and calcium to a lesser extent than furosemide
In trials of up to 48 weeks’ duration in patients with mild to moderate essential hypertension, torasemide, administered as a single daily dose, has been shown to achieve adequate blood pressure control reaching steady-state within 8 to 12 weeks. Those patients not responding initially have generally responded to a doubling of the dose. Comparative trials of up to 6 months show torasemide is as effective as indapamide, hydrochlorothiazide or a combination of triamterene/hydrochlorothiazide in maintaining control of blood pressure
Torasemide has also been used successfully to treat oedematous states associated with chronic congestive heart failure, renal disease and hepatic cirrhosis. In short term trials control of blood pressure, bodyweight and residual oedema has been sustained. Torasemide appears to be a useful alternative to furosemide in these patients, providing potent and long-lasting diuresis while being relatively potassium and calcium sparing
In clinical trials to date torasemide has been well tolerated with adverse effects of a mild, transient nature reported by only small numbers of patients. Changes in biochemical parameters have been common, including decreases in plasma sodium and potassium levels and increases in plasma creatinine and uric acid levels. These changes are typical of loop diuretics. No changes were clinically significant nor were clinically relevant changes noted in glucose metabolism, cholesterol or triglyceride levels or in haematological values
Thus, torasemide is an interesting new loop diuretic with potential use in the treatment of mild to moderate essential hypertension and of oedematous states in which diuretic therapy is warranted. Preliminary studies suggest it to be as efficacious as other diuretics in common use and to have some advantage over furosemide in duration of action and in effects on potassium and calcium. However, further long term trials in larger groups of patients are needed to delineate the place of torasemide in therapy fully, both as a single agent and in combination with other currently accepted drug regimens
Pharmacodynamic Properties
Torasemide is a potent loop diuretic of the furosemide type, which acts via blockade of active sodium and chloride reabsorption in the thick ascending limb of the loop of Henle, producing a rapid and pronounced diuresis and saluresis. Urinary volume and the excretion of sodium and chloride increase linearly with increasing dose, the pattern being similar to that found with an equipotent dose of furosemide. However, the duration of action with torasemide is longer and there is generally a smaller effect on the excretion of potassium. Secondary antidiuresis after torasemide may be less prominent than with other shorter-acting high-ceiling loop diuretics. Over-all a dose of 10 to 20mg torasemide is considered equivalent in diuretic potency to furosemide 40mg, bumetanide 1mg or piretanide 12mg
Adequate diuresis can be produced with torasemide in patients with chronic heart failure or advanced renal disease. In patients with cirrhotic liver disease the acute diuretic effect of torasemide is more pronounced and prolonged than that of furosemide, while the excretion of potassium, calcium and magnesium ions appears to be less
Single doses of torasemide 2.5 to 10mg administered to patients with mild to moderate essential hypertension produced α fall in mean diastolic blood pressure of 24 to 29mm Hg. In a multicentre study in patients with hypertension, torasemide treatment for 24 weeks did not affect serum potassium levels
Single doses of torasemide produce the increases in plasma renin activity, in angiotensin II and aldosterone levels, and in the urinary excretion of renal prostaglandin E and dopamine characteristic of loop diuretics, but no statistically significant effects have been reported in multiple-dose studies. No consistent effect on glomerular filtration rate has been observed
Pharmacokinetic Properties
Torasemide is rapidly absorbed following an oral dose; peak plasma concentration is achieved in the first hour. The pharmacokinetics of torasemide can be described by a 2-compartment open model. Both the plasma concentration of torasemide and the area under the plasma concentration-time curve (AUC) are linearly related to dose. The systemic availability of torasemide is in the range of 76 to 92%
The volume of distribution after a single intravenous dose of torasemide ranges from 0.14 to 0.19 L/kg, which approximates extracellular fluid volume. Plasma protein binding is estimated at 97 to 99%
The metabolism and elimination of torasemide involves hepatic biotransformation followed by both renal and nonrenal excretion of metabolites. Three main metabolites have been identified in humans: the M1 metabolite is about one-tenth as potent as torasemide, the M3 metabolite is equivalent in potency, and the M5 metabolite is inactive. Time to peak plasma concentration and elimination half-life of the metabolites are similar to those of the parent compound. However, the plasma concentration and urinary recovery of the two active metabolites are low and thus they are not of clinical importance in patients with normal renal and hepatic function
After a single oral dose of torasemide, the cumulative urinary excretion is comprised of 21% parent compound, 12% metabolite M1, 2% metabolite M3 and 34% inactive metabolite M5. The elimination half-life of torasemide and metabolites is in the range of 2 to 4 hours
Alterations in the pharmacokinetics of torasemide have not been demonstrated in elderly patients with normal renal and hepatic function or in patients with advanced renal disease
A linear correlation between torasemide dose and diuretic effect has been demonstrated over the range from 5 to 100mg with a flattening of the dose-response curve at higher doses. Urinary volume and the excretion of sodium and chloride are linearly related to dose
Therapeutic Use
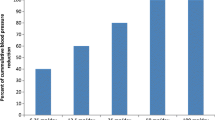
In a small number of double-blind parallel trials in patients with mild to moderate essential hypertension, torasemide in daily doses of 2.5 to 5mg over 12 to 24 weeks was as effective as indapamide 2.5 to 5mg, hydrochlorothiazide 25mg or fixed combinations of triamterene/hydrochlorothiazide 50/25mg or amiloride/hydrochlorothiazide 5/50mg in achieving and maintaining blood pressure control. Tolerability was good in all studies. The efficacy of torasemide has also been demonstrated in several noncomparative clinical trials with durations up to 48 weeks. Torasemide was administered as monotherapy usually in a single daily dose of 2.5 to 5mg. A reduction in diastolic blood pressure to ≤ 90mm Hg was achieved in 71 to 95% of patients with a dose of 2.5 or 5mg; in nonresponders, doubling of the dose produced a reduction to target blood pressure in 70 to 80% of patients. Tolerance to the antihypertensive effect of torasemide was not apparent
A beneficial effect of torasemide 10mg daily on pulmonary and cardiac haemodynamics in patients with previously untreated chronic heart failure has been demonstrated in a study over 4 weeks. Parameters of both preload and afterload were reduced. Torasemide 5 to 20mg daily, administered to patients with congestive heart failure who had been pretreated for 4 weeks with furosemide, was shown to reduce body weight significantly and to conserve the status of patients initially free of oedema throughout the 48-week study. Of patients who had residual oedema after furosemide pretreatment, 82% were free of oedema at the end of the trial. In controlled comparative trials, torasemide 5 to 10mg daily has been as effective as furosemide 40mg daily in improving clinical symptoms in patients with chronic congestive heart failure with oedema, while a daily dose of torasemide 20mg provided greater efficacy. A starting dose of torasemide 5mg was suggested from these trials
A number of short term trials have compared torasemide in daily doses of 100 to 400mg with furosemide 250 to 1000mg for the diuretic management of patients with advanced chronic renal failure. Torasemide was as effective as furosemide in promoting diuresis and preventing weight gain without adversely affecting neurological status
Finally, the combination of torasemide with spironolactone has been shown to be as effective as furosemide/spironolactone and more effective than spironolactone monotherapy in the diuretic management of patients with hydropically decompensated liver failure. Oedema and ascites tended to resolve with an associated improvement in liver enzyme values and neurological status
Further comparative studies in larger groups of patients over prolonged periods may be necessary to delineate fully the clinical profile of torasemide, alone and in combination, relative to other diuretics in common use for the treatment of hypertension and chronic cardiac, renal or hepatic disease
Adverse Effects
Adverse effects of torasemide are mild and transient in nature, occurring in only small numbers of patients and rarely necessitating treatment withdrawal. They include orthostatic hypotension, gastrointestinal disturbance, muscle cramps, dizziness, fatigue, headache and skin rash. Although potentially ototoxic as a high-ceiling loop diuretic, torasemide did not produce hearing loss when administered in daily doses of up to 400mg over a 4-week period to patients with renal disease
Drug Interactions
Nonsteroidal anti-inflammatory drugs (NSAIDs) have been shown to decrease the diuretic and natriuretic response to loop diuretics. Indomethacin pretreatment has been shown to reduce the excretion of water and sodium caused by single doses of torasemide, essentially under conditions of sodium restriction. Although the effect of long term treatment with torasemide in combination with probenecid or NSAIDs is unknown, the possibility of reduced diuresis should be considered, especially in patients requiring dietary sodium restriction
Dosage and Administration
In patients with mild to moderate essential hypertension a daily dose of torasemide of between 2.5 and 5mg (in some cases up to 10mg) may be used. Incremental blood pressure reduction may continue for up to 8 weeks. In the treatment of patients with chronic congestive heart failure a suitable starting dose is 5mg daily with increments to 10 or 20mg as needed. In patients with advanced chronic renal failure a daily dose of torasemide 100 to 200mg has been used successfully without adverse effects. Dosage adjustment is not necessary in patients undergoing haemodialysis or haemofiltration. In patients with cirrhotic liver disease, torasemide can be used in daily doses of 10 to 20mg, in combination with an aldosterone antagonist to promote diuresis
Similar content being viewed by others
References
Achhammer I, Eberhard R. Comparison of serum potassium levels during long-term treatment of hypertensive patients with 2.5mg torasemide/day or 50mg triamterene/25mg hydrochlorothiazide/day. In Krück et al. (Eds) Torasemide: clinical pharmacology and therapeutic applications. Proceedings of the 2nd International Symposium on Torasemide, Munich, October 21–23, 1988. Progress in Pharmacology and Clinical Pharmacology, Vol. 8, No. 1, pp. 211–220, Fischer Verlag, Stuttgart, New York, 1990
Achhammer I. Long term efficacy and tolerance of torasemide in congestive heart failure. In Krück et al. (Eds) Torasemide: clinical pharmacology and therapeutic applications. Proceedings of the 2nd International Symposium on Torasemide, Munich, October 21–23, 1988. Progress in Pharmacology and Clinical Pharmacology, Vol. 8, No. 1, pp. 127–136, Fischer Verlag, Stuttgart, New York, 1990
Ambroes Y, Ronflette I, Dodion L. Diuretic activity, safety and pharmacokinetics of torasemide during chronic treatment in normal subjects. European Journal of Clinical Pharmacology 31 (Suppl.): 1–7, 1986
Andreucci YE, Russo D, Memoli B, Testa A, Rampino T, et al. Efficacy of IV torasemide in the treatment of acute and chronic high grade renal failure. In Krück et al. (Eds) Torasemide: clinical pharmacology and therapeutic applications. Proceedings of the 2nd International Symposium on Torasemide, Munich, October 21–23, 1988. Progress in Pharmacology and Clinical Pharmacology, Vol. 8, No. 1, pp. 229–238, Fischer Verlag, Stuttgart, New York, 1990
Barr WH, Smith HL, Karnes HT, Sica D, Vetticaden SJ, et al. Torasemide dose-proportionality of pharmacokinetics and pharmacodynamics. In Krück et al. (Eds) Torasemide: clinical pharmacology and therapeutic applications. Proceedings of the 2nd International Symposium on Torasemide, Munich, October 21–23, 1988. [Progress in Pharmacology and Clinical Pharmacology, Vol. 8, No. 1, pp. 29–38, Fischer Verlag, Stuttgart, New York, 1990a
Barr WH, Smith HL, Karnes HT, Sica P, Vetticaden S, et al. Comparison of bioavailability, pharmacokinetics and pharmacodynamics of torasemide in young and elderly healthy volunteers. In Krück et al. (Eds) Torasemide: clinical pharmacology and therapeutic applications. Proceedings of the 2nd International Symposium on Torasemide, Munich, October 21–23, 1988. Progress in Pharmacology and Clinical Pharmacology, Vol. 8, No. 1, pp. 15–28, Fischer Verlag, Stuttgart, 1990b
Baumgart P, Walger P, van Eiff M, Achhammer I. Long-term efficacy and tolerance of torasemide in essential hypertension. In Krück et al. (Eds) Torasemide: clinical pharmacology and therapeutic applications. Proceedings of the 2nd International Symposium on Torasemide, Munich, October 21–23, 1988. Progress in Pharmacology and Clinical Pharmacology, Vol. 8, No 1, pp. 169–182, Fischer Verlag, Stuttgart, New York, 1990
Besenfelder E. The determination of torasemide and metabolites in plasma by high-performance liquid chromatography. Journal of Pharmaceutical and Biomedical Analysis 5: 259–266, 1987
Brater DC. Clinical pharmacology of loop diuretics. Drugs 41 (Suppl.), in press, 1991
Brater DC, Leinfelder J, Anderson SA. Clinical pharmacology of torasemide, a new loop diuretic. Clinical Pharmacology and Therapeutics 42: 187–192, 1987
Broekhuysen J, Deger F, Douchamps J, Ducarne H, Herchuelz A. Torasemide, a new potent diuretic. Double-blind comparison with furosemide. European Journal of Clinical Pharmacology 31 (Suppl.): 29–34, 1986
Brunner G, Chang J, Gonzales J, Piesche L. Treatment of cirrhotic ascites/edema: a therapeutic approach with torasemide-spironolactone. In Puschett JB, Greenberg A (Eds) Diuretics: chemistry, pharmacology, and clinical applications. Proceedings of the Third International Conference on Diuretics, Mexico City, Mexico, April 2–7, 1989. pp. 402–404, Elsevier, New York, 1990
Brunner G, von Bergmann K, Häcker W, von Möllendorff E. Comparison of diuretic effects and pharmacokinetics of torasemide and furosemide after a single oral dose in patients with hydropically decompensated cirrhosis of the liver. Arzneimittel-Forschung/Drug Research 38: 176–179, 1988
Clasen W, Khartabil T, Imm St, Kindler J. Torasemide for diuretic treatment of advanced chronic renal failure. Arzneimittel-Forschung/Drug Research 38: 209–211, 1988
Clerckx-Braun F, Lesne M. Pharmacokinetic study of a new diuretic: torasemide in man. Journal de Pharmacie de Belgique 35: 223–224, 1980
Crippa G, Cassi A, Vergani F. Comparison of the acute diuretic effect of torasemide and furosemide in healthy subject and in cirrhotic patient. In Puschett JB, Greenberg A (Eds) Diuretics: chemistry, pharmacology, and clinical applications. Proceedings of the Third International Conference on Diuretics, Mexico City, Mexico, April 2–7, 1989. pp. 396–398, Elsevier, New York, 1990
Cuvelier R, Pellegrin P, Lesne M, van Ypersele de Strihou Ch. Site of action of torasemide in man. European Journal of Clinical Pharmacology 31 (Suppl.): 15–19, 1986
Delarge J. Chemistry and pharmacological properties of the pyridine-3-sulfonylurea derivative torasemide. Arzneimittel-Forschung/Drug Research 38: 144–150, 1988
Dodion L, Ambroes Y, Lameire N. A comparison of the pharmacokinetics and diuretic effects of two loop diuretics, torasemide and furosemide, in normal volunteers. European Journal of Clinical Pharmacology 31 (Suppl.): 21–27, 1986
Dodion L, Willems JL. Study of the elimination kinetics of torasemide, a novel loop diuretic, in renal insufficiency. European Journal of Clinical Pharmacology 32 (Suppl.): 49–51, 1986
Dupont AG. Low dose therapy in hypertension. Abstract. Third International Conference on Diuretics, Mexico City, Mexico, April 2–7, 1989
Dupont AG, Gerlo E, Van der Niepen P, Laekeman G, Piepsz A. Renal pharmacodynamic effects of torasemide and furosemide in normal man. Arzneimittel-Forschung/Drug Research 38: 172–175, 1988a
Dupont AG, Schoors D, Six RO, Vanhaelst L. Antihypertensive efficacy of low dose torasemide in essential hypertension: a placebo-controlled study. Journal of Human Hypertension 2: 265–268, 1988b
Düsing R, Piesche L. Second line therapy of congestive heart failure with torasemide. In Krück et al. (Eds) Torasemide: clinical pharmacology and therapeutic applications. Proceedings of the 2nd International Symposium on Torasemide, Munich, October 21–23, 1988. Progress in Pharmacology and Clinical Pharmacology, Vol. 8, No. 1, pp. 105–120, Fischer Verlag, 1990
Elia G, Ferrari C, Degli Antoni A, Penna A, Fiaccadori F. A short-term clinical study of a new loop diuretic, torasemide, in cirrhosis. Italian Journal of Gastroenterology 21: 324–328, 1989
Fiehring H, Achhammer I. Influence of 10mg torasemide i.v. and 20mg furosemide i.v. on the intracardiac pressures in patients with heart failure at rest and in exercise. In Krück et al. (Eds) Torasemide: clinical pharmacology and therapeutic applications. Proceedings of the 2nd International Symposium on Torasemide, Munich, October 21–23, 1988. Progress in Pharmacology and Clinical Pharmacology, Vol. 8, No. 1, pp. 97–104, Fischer Verlag, Stuttgart, New York, 1990
Ghys A, Denef J, Delarge J, Georges A. Renal effects of the high ceiling diuretic torasemide in rats and dogs. Arzneimittel-Forschung/Drug Research 35: 1527–1531, 1985a
Ghys A, Denef J, de Suray JM, Gerin M, Georges A, et al. Pharmacological properties of the new potent diuretic torasemide in rats and dogs. Arzneimittel-Forschung/Drug Research 35: 1520–1526, 1985b
Grabensee B. Efficacy of oral torasemide (TS) and furosemide (FS) in the treament of chronic renal failure. Abstract. 2nd International Symposium on Torasemide, Munich, October 21–23, 1988
Greger R. Diuretika — Hemmer der Elektrolytresorption im Nephron. Arzneimitteltherapie 11: 308–315, 1989
Greger R. Modes of action of diuretics. In Knauf H & Mutschier E (Eds) Diuretika — Prinzipien der Klinischen Anwendung [Principles of the clinical use of diuretics], Section 1, pp. 3–17, Urban & Schwarzenberg, Munich, 1986
Greger R. Inhibition of active NaCl reabsorption in the thick ascending limb of the loop of Henle by torasemide. Arzneimittel-Forschung/Drug Research 38: 151–152, 1988
Greger R, Wangemann P, Wittner M, di Stefano A, Lang HJ, et al. Blockers of active transport in the thick ascending limb of the loop of Henie. In Andreucci VE & Dal Canton A (Eds) Diuretics: basic, pharmacological and clinical aspects. Proceedings of the International Meeting on Diuretics, Sorrento, Italy, May 26–30, 1986, pp. 33–38, Martinus Nijhoff Publishing, Boston, 1987
Herchuelz A, Deger F, Douchamps J, Ducarne H, Broeckhuysen J. Comparative pharmacodynamics of torasemide and furosemide in patients with oedema. Arzneimittel-Forschung/Drug Research 38: 180–183, 1988
Herchuelz A, Derenne F, Deger F, Juvent M, Van Ganse E, et al. Interaction between nonsteroidal anti-inflammatory drugs and loop diuretics: modulation by sodium balance. J. Pharmacol. Exp. Therap. 248: 1175–1181, 1989
Hermes H, Heidenreich O. Renal effects of torasemide in the rat. Clearance and micropuncture studies. Arzneimittel-Forschung/ Drug Research 35: 1532–1535, 1985
Isbary J, Achhammer I, Wetzeis E. The influence of torasemide 20mg iv and furosemide 20mg iv on haemodynamics and diuresis in patients with high grade left heart failure. In Krück et al. (Eds) Torasemide: clinical pharmacology and therapeutic applications. Proceedings of the 2nd International Symposium on Torasemide, Munich, October 21–23, 1988. Progress in Pharmacology and Clinical Pharmacology, Vol. 8, No. 1, pp. 137–146, Fischer Verlag, Stuttgart, New York, 1990
Karnes HT, Farthing D, Besenfelder E. Solid phase extraction with automated elution and HPLC of torsemide and metabolites from plasma. Journal of Liquid Chromatography 12: 1809–1818, 1989
Klinke R, Mertens M. Quantitative assessment of torasemide ototoxicity. Arzneimittel-Forschung 38: 153–155, 1988
Klütsch K, Grosswendt J, Haecker W. Single dose comparison of torasemide and furosemide in patients with advanced renal failure. Arzneimittel-Forschung 38: 200–204, 1988
Knauf H, Mutschier E. Saluretic effect of the loop diuretic torasemide in chronic renal failure. European Journal of Clinical Pharmacology 39: 337–343, 1990
Krämer BK, Ress KM, von Moellendorff E, Piesche L, Achhammer I, et al. Influence of torasemide on serum level and renal elimination of digoxin in healthy volunteers. In Krück et al. (Eds) Torasemide: clinical pharmacology and therapeutic applications. Proceedings of the 2nd International Symposium on Torasemide, Munich, October 21–23, 1988. Progress in Pharmacology and Clinical Pharmacology, Vol. 8, No. 1, pp. 39–46, Fischer Verlag, Stuttgart, New York, 1990
Kult J, Hacker W, Glocke M. Comparison of efficacy and tolerance of different oral doses of torasemide and furosemide in patients with advanced chronic renal failure. Arzneimittel-Forschung/Drug Research 38: 212–214, 1988
Kult J, Ziegler J, von Möllendorff E. Pharmacodynamics and — kinetics of torasemide and furosemide in patients with high grade renal failure after: administration of high intravenous doses. In Krück et al. (Eds) Torasemide: clinical pharmacology and therapeutic applications. Proceedings of the 2nd International Symposium on Torasemide, Munich, October 21–23, 1988. Progress in Pharmacology and Clinical Pharmacology, Vol. 8, No. 1, pp. 239–248, Fischer Verlag, Stuttgart, New York, 1990
Lambe R, Kennedy O, Kenny M, Darragh A. Study of the tolerance and diuretic properties of torasemide following oral or intravenous administration to healthy volunteers. European Journal of Clinical Pharmacology 31 (Suppl.): 9–14, 1986
Langbehn A-F, Achhammer I, Bölke T. Acute hemodynamic effects of 20 mg torasemide and 20 mg furosemide given intravenously to patients with congestive heart failure. In Krück et al. (Eds) Torasemide: clinical pharmacology and therapeutic applications. Proceedings of the 2nd International Symposium on Torasemide, Munich, October 21–23, 1988. Progress in Pharmacology and Clinical Pharmacology, Vol. 8, No. 1, pp. 147–155, Fischer Verlag, Stuttgart, New York, 1990
Lesne M. Comparison of the pharmacokinetics and pharmacodynamics of torasemide and furosemide in healthy volunteers. Arzneimittel-Forschung/Drug Research 38: 160–163, 1988
Lesne M, Clerckx-Braun F Duhoux P, van Ypersele de Strihou C. Pharmacokinetic study of torasemide in humans: an overview of its diuretic effect. International Journal of Clinical Pharmacology, Therapeutics and Toxicology 20: 382–387, 1982
Loute G, Adam A, Ers P, Heremans C, Willems B. The influence of haemodialysis and haemofiltration on the clearance of torasemide in renal failure. European Journal of Clinical Pharmacology 31 (Suppl.): 53–55, 1986
Lupinacci L, Puschett JB. An examination of the site and mechanism of action of torasemide in man. Journal of Clinical Pharmacology 28: 441–447, 1988
Mourad G, Haecker W, Mion C. Dose-dependent salidiuretic efficacy of torasemide in comparison to furosemide and placebo in advanced renal failure. Arzneimittel-Forschung/Drug Research 38: 205–208, 1988
Mueller G, Haecker W. Torasemide, a new loop diuretic in the treatment of essential hypertension without renal impairment. Abstract no. 13. Cardiovascular Pharmacotherapy International Symposium, 1985
Neugebauer G, Besenfelder E, von Möllendorff E. Pharmacokinetics and metabolism of torasemide in man. Arzneimittel-Forschung/Drug Research 38: 164–166, 1988
Piesche L, Achhammer I, Glocke M, Haecker W. Comparison of torasemide with furosemide in patients with chronic heart failure. In Puschett JB & Greenberg A (Eds) Diuretics II. Chemistry, pharmacology and clinical applications, pp. 65–67, Elsevier Science Publishing Co. Inc., 1987
Podszus T, Piesche L. Effect of torasemide on pulmonary and cardiac haemodynamics after oral treatment of chronic heart failure. In Krück et al. (Eds) Torasemide: clinical pharmacology and therapeutic applications. Proceedings of the 2nd International Symposium on Torasemide, Munich, October 21–23,1988. Progress in Pharmacology and Clinical Pharmacology, Vol. 8, No. 1, pp. 158–166, Fischer Verlag, 1990
Prasad VK, Vetticaden SJ, Purich ED. Dose proportionality study of torasemide, a new diuretic — pharmacodynamic effects. J. Pharmaceut. Sci. 76: Abstract D 07-W-32, 1987
Reyes AJ, Chiesa PD, Santucci MR, Batista LB, Olhaberry JV, et al. Hydrochlorothiazide versus a non-diuretic dose of torasemide as once-daily antihypertensive monopharmacotherapy in elderly patients. A randomized and double-blind study. In Krück et al. (Eds) Torasemide: clinical pharmacology and therapeutic applications. Proceedings of the 2nd International Symposium on Torasemide, Munich, October 21–23, 1988. Progress in Pharmacology and Clinical Pharmacology, Vol. 8, No. 1, pp. 183–210, Fischer Verlag, 1990a
Reyes AJ, Leary WP, van der Byl K. Excretions of urinary fluid and solutes after single doses of furosemide and hydrochlorothiazide and of four different single doses of the diuretic torasemide in healthy subjects. In Krück et al. (Eds) Torasemide: clinical pharmacology and therapeutic applications. Proceedings of the 2nd International Symposium on Torasemide, Munich, October 21–23, 1988. Progress in Pharmacology and Clinical Pharmacology, Vol. 8, No. 1, pp. 47–72, Fischer Verlag, 1990b
Russo D, Gazzotti RM, Testa A. Torasemide, a new loop diuretic, in patients with chronic renal failure. Nephron 55: 141–145, 1990
Scheen AJ. Dose-response curve for torasemide in healthy volunteers. Arzneimittel-Forschung/Drug Research 38: 156–159, 1988
Scheen AJ, Vancrombreucq JC, Delarge J, Luyckx AS. Diuretic activity of torasemide and furosemide in chronic heart failure: a comparative double blind cross-oyer study. European Journal of Clinical Pharmacology 31 (Suppl.): 35–42, 1986
Schlatter E. The current view of the function of the thick ascending limb. In Persson AEG & Boberg U (Eds) The juxtaglomerular apparatus, Chapter 4, pp. 53–62, Elsevier Science Publishers (Biomedical Division), 1988
Schulz W, Dörfler A, Stiehl L, Achhammer I. Double-blind clinical trial investigating the efficacy and long-term tolerance of torasemide 200mg p.o. compared with furosemide 500mg p.o. and placebo p.o. in patients with chronic renal failure on hemodialysis, a multicentre study. In Krück et al. (Eds) Torasemide: clinical pharmacology and therapeutic applications. Proceedings of the 2nd International Symposium on Torasemide, Munich, October 21–23, 1988. Progress in Pharmacology and Clinical Pharmacology, Vol. 8, No. 1, pp. 249–258, Fischer Verlag, Stuttgart, New York, 1990
Spahn H, Knauf H, Mutschler E. Pharmacodynamics and kinetics of torasemide and its metabolites in chronic renal failure after IV administration of 20mg torasemide. In Andreucci VE & Dal Canton A (Eds) Diuretics: basic, pharmacological and clinical aspects, pp. 376–378, Martinus Nijhoff Publishing, Boston, 1986
Spahn H, Knauf H, Mutschler E. Pharmacokinetics of torasemide and its metabolites in healthy controls and in chronic renal failure. Europ. J. Clin. Pharmacol. 39: 345–348, 1990
Spannbrucker N, Achhammer I, Metz P, Glocke M. Comparative study on the antihypertensive efficacy of torasemide and indapamide in patients with essential hypertension. Arzneimittel-Forschung/Drug Research 38: 190–193, 1988
Spieker C, Zidek W, Häcker W, Schmidt W, Vetter H. Assessment of intracellular sodium and calcium in essential hypertension during diuretic treatment. Arzneimittel-Forschung/Drug Research 38: 188–190, 1988
Spieker C, Zidek W, Vetter H. Acute effect of torasemide on the renin activity and on intracellular electrolytes in hypertensive patients. In Krück et al. (Eds) Torasemide: clinical pharmacology and therapeutic applications. Proceedings of the 2nd International Symposium on Torasemide, Munich, October 21–23, 1988. Progress in Pharmacology and Clinical Pharmacology, Vol. 8, No. 1, pp. 221–226, Fischer Verlag, Stuttgart, 1990
Stauch M, Stiehl L. Controlled, double-blind clinical trial on the efficacy and tolerance of torasemide in comparison with furosemide in patients with congestive heart failure — a multicenter study. In Krück et al. (Eds) Torasemide: clinical pharmacology and therapeutic applications. Proceedings of the 2nd International Symposium on Torasemide, Munich, October 21–23,1988. Progress in Pharmacology and Clinical Pharmacology, Vol. 8, No. 1, pp. 121–126, Fischer Verlag, New York, 1990
Stolear IC, Achhammer I, Georges B. Efficacy of torasemide in the treatment of patients with high grade renal failure on dialysis. In Krück et al. (Eds) Torasemide: clinical pharmacology and therapeutic applications. Proceedings of the 2nd International Symposium on Torasemide, Munich, October 21–23,1988. Progress in Pharmacology and Clinical Pharmacology, Vol. 8, No. 1, pp. 259–268, Fischer Verlag, Stuttgart, New York, 1990
Stroobandt R, Dodion L, Kesteloot H. Clinical efficacy of torasemide, a new diuretic agent, in patients with acute heart failure: a double blind comparison with furosemide. Archives Internationales de Pharmacodynamie 260: 151–158, 1982
Thurau K, Schnermann J. Die Natriumkonzentration an den Macula densa-Zellen als regulierender Faktor für das Glomerulumfiltrat (Mikropunctionsversuche). Klinische Wochenschrift 43: 410–413,1965
van Ganse E, Douchamps J, Deger F, Staroukine M, Verniory A, et al. Failure of indomethacin to impair the diuretic and natriuretic effects of the loop diuretic torasemide in healthy volunteers. European Journal of Clinical Pharmacology 31 (Suppl.): 43–47, 1986
von Möllendorff E, Neugebauer G. Pharmacokinetics of torasemide in patients with congestive heart failure. In Krück et al. (Eds) Torasemide: clinical pharmacology and therapeutic applications. Proceedings of the 2nd International Symposium on Torasemide, Munich, October 21–23, 1988. Progress in Pharmacology and Clinical Pharmacology, Vol. 8, No. 1, pp. 73–80, Fischer Verlag, Stuttgart, New York, 1990
Wittner M, Di Stefano A, Schlatter E, Delarge J, Greger R. Torasemide inhibits NaCl reabsorption in the thick ascending limb of the loop of Henle. Pflügers Archiv/European Journal of Physiology 407: 611–614, 1986
Wittner M, Di Stefano A, Wangemann P, Greger R. How do loop diuretics act? Drugs 41 (Suppl.), in press, 1991
Zanazzi VA, Ratti G, Ballocchi S, Scarpioni LL. Torasemide versus furosemide in cirrhosis. In Puschett JB, Greenberg A (Eds) Diuretics: chemistry, pharmacology and clinical applications. Proceedings of the Third International Conference on Diuretics, Mexico City, Mexico, April 2–7, 1989, pp. 399–401, Elsevier, New York, 1990
Author information
Authors and Affiliations
Additional information
Various sections of the manuscript reviewed by: V.E. Andreucci, Department of Nephrology, Second Faculty of Medicine, University of Naples, Naples, Italy; D.C. Brater, Clinical Pharmacology Division, School of Medicine, Indiana University, Indianapolis, Indiana, USA; G. Brunner, Medizinische Hochschule Hannover, Hannover, Federal Republic of Germany; W. Clasen, Krankenhaus der Missionsschwestern, Münster Hiltrup, Federal Republic of Germany; A.G. Dupont, Faculteit Geneeskunde en Farmacie, Vrije Universiteit Brüssel, Brussels, Belgium; A. Ebihara, Department of Clinical Pharmacology, Jichi Medical School, Tochigi, Japan; R. Greger, Institut für Physiologie, Universität Freiburg, Freiburg, Federal Republic of Germany; A. Herchuelz, Faculté de Médecine, Université Libre de Bruxelles, Brussels, Belgium; W.F. Keane, Department of Medicine, University of Minnesota, Minneapolis, Minnesota, USA; A. Lant, Department of Clinical Pharmacology and Therapeutics, Charing Cross and Westminster Medical School, University of London, London, England; T.O. Morgan, Department of Physiology, University of Melbourne, Melbourne, Victoria, Australia
Rights and permissions
About this article
Cite this article
Friedel, H.A., Buckley, M.MT. Torasemide. Drugs 41, 81–103 (1991). https://doi.org/10.2165/00003495-199141010-00008
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199141010-00008