Abstract
Background: 3,4-Methylenedioxymethamphetamine (MDMA) is a synthetic amphetamine derivative typically used for recreational purposes. The participation of cytochrome P450 (CYP) 2D6 in the oxidative metabolism of MDMA may suggest an increased risk of acute toxicity in CYP2D6 poor metabolisers. This study was aimed at assessing the contribution of CYP2D6 to MDMA disposition in vivo using paroxetine as a metabolic probe inhibitor. Paroxetine, a CYP2D6 inhibitor, was repeatedly administered before MDMA administration.
Study design: This was a randomised, double-blind, crossover, placebo-controlled trial conducted in seven healthy male volunteers who were CYP2D6 extensive metabolisers. Treatment conditions (paroxetine/MDMA and placebo/ MDMA) were randomly assigned. Each volunteer participated in two 3-day sessions. On days 1, 2 and 3 subjects received a single oral dose of paroxetine or placebo 20mg. On the third day, a single oral dose of MDMA 100mg was administered in both paroxetine and placebo conditions.
Methods: Plasma concentration-time profiles and urinary recoveries of MDMA and its metabolites were measured, as well as plasma concentrations of paroxetine, (3S,4R)-4-(4-fluorophenyl)-3-(3,4-methylenedioxyphenoxymethyl)-piperidine, and (3S,4R)-4-(4-fluorophenyl)-3-(3-methoxy-4-hydroxyphenoxymethyl)-piperidine (HM-paroxetine).
Results: Paroxetine given before MDMA resulted in significant increases of MDMA area under the plasma concentration-time curve from 0 to 27 hours (AUC27) [23%], AUC from zero to infinity (AUC∞) [27%] and maximum plasma concentration (Cmax) [17%], without significant differences in MDMA time to reach Cmax (tmax). MDMA elimination-related pharmacokinetic parameters showed a significant reduction of MDMA elimination rate constant (Ke) [−14%] and plasmatic clearance (CLP) [−29%]. In the case of 3,4-dihydroxymethamphetamine (HHMA), a 21% decrease in Cmax with no significant differences in AUC27, AUC∞, Ke and elimination half-life) were found. 4-Hydroxy-3-methoxymethamphetamine (HMMA) showed a decrease in plasma concentrations with a reduction in AUC27 (−28%), AUC∞ (−20%) and Cmax (−46%). In the case of 3,4-methylenedioxyamphetamine (MDA) an increase in Cmax (17%) and AUC27 (16%) was found. Following paroxetine pretreatment, the urinary recovery (0–45 hours) of MDMA increased by 11%; HHMA and HMMA urinary recoveries were 27% and 16% lower, respectively compared with placebo. The ratio of Cmax values of paroxetine and its metabolite on days 1 and 3 showed a 3-fold reduction, with no differences in tmax.
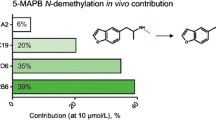
Discussion and conclusion: The contribution of CYP2D6 to MDMA metabolism in humans is not >30%, therefore other CYP isoenzymes may contribute to O-demethylenation of MDMA. Accordingly, the relevance of genetic polymorphism in CYP2D6 activity on MDMA effects and MDMA-induced acute toxicity should be examined as well as the interactions of other CYP2D6 substrates with MDMA, once the enzyme is inhibited. The pharmacokinetics of HM-paroxetine in humans after the administration of repeated doses is reported for the first time in this study.






Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Mas M, Farré M, de la Torre R, et al. Cardiovascular and neuroendocrine effects and pharmacokinetics of 3,4-methylenedioxymethamphetamine in humans. J Pharmacol Exp Ther 1999 Jul; 290(1): 136–45
Parrott AC. Human psychopharmacology of ecstasy (MDMA): a review of 15 years of empirical research. Hum Psychopharmacol 2001 Dec; 16(8): 557–77
McCann UD, Slate SO, Ricaurte GA. Adverse reactions with 3,4-methylenedioxymethamphetamine (MDMA; ‘ecstasy’). Drug Saf 1996 Aug; 15(2): 107–15
Morgan MJ. Ecstasy (MDMA): a review of its possible persistent psychological effects. Psychopharmacology (Berl) 2000 Oct; 152(3): 230–48
McCann UD, Eligulaschvili V, Ricaurte GA. (±) 3,4-Methylendioxymethamphetamine (’ecstasy’)-induced serotonin neurotoxicity: clinical studies. Neuropsychobiology 2000; 42(1): 11–6
McCann UD, Szabo Z, Scheffel U, et al. Positron emission tomographic evidence of toxic effect of MDMA (’ecstasy’) on brain serotonin neurons in humans beings. Lancet 1998 Oct 31; 352(9138): 1433–7
McCann UD, Ridenour A, Shaham Y, et al. Serotonin neurotoxicity after (±) 3,4-methylenedioxymethamphetamine (MDMA; ‘ecstasy’): a controlled study in humans. Neuropsychopharmacology 1994 Apr; 10(2): 129–38
Gill JR, Hayes JA, DeSouza IS, et al. Ecstasy (MDMA) deaths in New York City: a case series and review of the literature. J Forensic Sci 2002 Jan; 47(1): 121–6
Tucker GT, Lennard MS, Ellis SW, et al. The demethylenation of the methylenedioxymethamphetamine (’ecstasy’) by debrisoquine hydroxylase (CYP2D6). Biochem Pharmacol 1994; 47(7): 1151–6
Kreth K, Kovar K, Schwab M, et al. Identification of the human cytochromes P450 involved in the oxidative metabolism of ‘ecstasy’-related designer drugs. Biochem Pharmacol 2000 Jun 15; 59(12): 1563–71
Kraemer T, Maurer HH. Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives. Ther Drug Monit 2002 Apr; 24(2): 277–89
de la Torre R, Farré M, Roset PN, et al. Pharmacology of MDMA in humans. Ann NY Acad Sci 2000; 914: 225–37
Segura M, Ortuño J, Farré M, et al. 3,4-dihydroxymethamphetamine (HHMA): a major in vivo 3,4-methylene-dioxymethamphetamine (MDMA) metabolite in humans. Chem Res Toxicol 2001 Sep; 14(9): 1203–8
Hiramatsu M, Kumagai Y, Unger SE, et al. Metabolism of methylenedioxymethamphetamine: formation of dihydroxymethamphetamine and a quinone identified as its glutathione adduct. J Pharmacol Exp Ther 1990 Aug; 254: 521–7
Bai F, Lau SS, Monks TJ. Glutathione and N-acetylcysteine conjugates of alpha-methyldopamine produce serotonergic neurotoxicity: possible role in methylenedioxyamphetamine-mediated neurotoxicity. Chem Res Toxicol 1999 Dec; 12(12): 1150–7
Monks TJ, Jones DC. The metabolism and toxicity of quinones, quinonimines, quinone methides, and quinone-thioethers. Curr Drug Metab 2002 Aug; 3(4): 425–38
Baggott M, Heifets B, Jones RT, et al. Chemical analysis of ecstasy pills. JAMA 2001 Jan 24–31; 285(4): 409–10
McCann UD, Ricaurte GA. Reinforcing subjective effects of (±) 3,4 methylendioxymethamphetamine (’ecstasy’) may be separable from its neurotoxic actions: clinical evidence. J Clin Psychopharmacol 1993 Jun; 13(3): 214–7
Anonymous. Ecstasy and fluoxetine [letter]. Pharmaceutical J 1995; 255: 611
Stein DJ, Rink J. Effects of ‘ecstasy’ blocked by serotonin reuptake inhibitors [letter]. J Clin Psychiatry 1999 Jul; 60(7): 485
Lauerma H, Wuorela M, Halme M. Interaction of serotonin reuptake inhibitor and 3,4-methylenedioxymethamphetamine [letter]. Biol Psychiatry 1998 Jun 15; 43(12): 929
Liechti ME, Wollenweider FX. Which neuroreceptors mediate the subjective effects of MDMA in humans: a summary of mechanistic studies. Hum Psychopharmacol 2001 Dec; 16(8): 589–98
Windhaber J, Maierhofer D, Dantendorfer K. Panic disorder induced by large doses of 3,4-methylenedioxymethamphetamine resolved by paroxetine. J Clin Psychopharmacol 1998 Feb; 18(1): 95–6
Sills TL, Greenshaw AJ, Baker GB, et al. Acute fluoxetine treatment potentiates amphetamine hyperactivity and amphetamine-induced nucleus accumbens dopamine release: possible pharmacokinetic interaction. Psychopharmacology 1999 Feb; 141(4): 421–7
Jeppesen U, Gram LF, Vistisen K, et al. Dose-dependent inhibitor of CYP1A2, CYP2C19 and CYP2D6 by citalopram, fluoxetine, fluvoxamine and paroxetine. Eur J Clin Pharmacol 1996; 51(1): 73–8
Preskorn SH. Clinically relevant pharmacology of selective serotonin reuptake inhibitors: an overview with emphasis on pharmacokinetics and effects on oxidative drug metabolism. Clin Pharmacokinet 1997; 32 Suppl. 1: 1–21
Wu D, Otton SV, Inaba T, et al. Interactions of amphetamine analogs with human liver CYP2D6. Biochem Pharmacol 1997 Jun 1; 53(11): 1605–12
Bloomer JC, Woods FR, Haddock RE, et al. The role of cytochrome P4502D6 in the metabolism of paroxetine by human liver microsomes. Br J Clin Pharmacol 1992 May; 33(5): 521–3
Kaye CM, Haddock RE, Langley PF, et al. A review of the metabolism and pharmacokinetics of paroxetine in man. Acta Psychatr Scand Suppl 1989; 350: 60–75
Gunasekara NS, Noble S, Benfield P. Paroxetine: an update of its pharmacology and therapeutic use in depression and a review of its use in other disorders. Drugs 1998 Jan; 55(1): 85–120
Tulloch IF, Johnson AM. Pharmacologic profile of paroxetine, a new selective serotonin reuptake inhibitor. J Clin Psychiatry 1992 Feb; 53 Suppl. 2: 7–12
Segura M, Ortuño J, Farré M, et al. Quantitative determination of paroxetine and its 4-hidroxy-3-methoxy metabolite in plasma by high performance liquid chromatography/electrospray ion trap mass spectrometry: application to pharmacokinetic studies. Rapid Commun Mass Spectrom 2003 Jul; 17(13): 1455–61
Bourin M, Chue P, Guillon Y. Paroxetine: a review. CNS Drug Rev 2001; 7(1): 25–47
Lin LY, DiStefano EW, Schmitz DA, et al. Oxidation of methamphetamine and methylenedioxymethamphetamine by CYP2D6. Drug Metab Dispos 1997 Sep; 25(9): 1059–64
de la Torre R, Farré M, Ortuño J, et al. Non-linear pharmacokinetics of MDMA (’ecstasy’) in humans. Br J Clin Pharmacol 2000 Feb; 49(2): 104–9
Baumann P. Pharmacokinetic-pharmacodynamic relationship of the selective serotonin reuptake inhibitors. Clin Pharmacokinet 1996 Dec; 31(6): 444–69
Delaforge M, Jaouen M, Bouille G. Inhibitory metabolite complex formation of methylenedioxymethamphetamine with rat and human cytochrome P450: particular involvement of CYP 2D. Environ Toxicol Pharmacol 1999; 7: 153–8
Bertelsen KM, Venkatakrishnan K, Von Moltke LL, et al. Apparent mechanism-based inhibition of human CYP2D6 in vitro by paroxetine: comparison with fluoxetine and quinidine. Drug Metab Dispos 2003 Mar; 31(3): 289–93
Hodgson E, Rose RL, Ryu DY, et al. Pesticide-metabolizing enzymes. Toxicol Lett 1995 Dec; 82–83: 73–81
Murray M. Mechanisms of inhibitory and regulatory effects of methylenedioxyphenyl compounds on cytochrome P450-dependent drug oxidation. Curr Drug Metab 2000 Jul; 1(1): 67–84
Schmidt B, Bircher J, Preisig R, et al. Polymorphic dextromethorphan metabolism: co-segregation of oxidative o-demethylation with debrisoquin hydroxylation. Clin Pharmacol Ther 1985; 38: 618–24
Pizarro N, Ortuño J, Farré M, et al. Determination of MDMA and its metabolites in blood and urine by gas chromatography-mass spectrometry and analysis of enantiomers by capillary electrophoresis. J Anal Toxicol 2002 Apr; 26(3): 157–65
Segura M, Ortuño J, McLure JA, et al. High-performance liquid chromatography with electrochemical detection applied to the analysis of 3,4-dihydroxymethamphetamine in human plasma and urine. J Chromatogr B 2002 Apr 5; 769(2): 313–21
Pizarro N, de la Torre R, Farré M, et al. Synthesis and capillary electrophoretic analysis enantiomerically enriched reference standards of MDMA and its main metabolites. Bioorg Med Chem 2002 Apr; 10(4): 1085–92
Segura M, Roura L, de la Torre R, et al. Synthesis of the major metabolite of paroxetine. Bioorg Chem 2003 Jul; 31(3): 248–58
Rowland M, Tozer TN. Intravenous dose. In: Rowland M, Tozer TN, editors. Clinical pharmacokinetics: concepts and applications. Philadelphia (PA): Lea & Febiger, 1989: 9–16
Usansky JI, Desai A, Tang-Liu D. Pharmacokinetic and pharmacodynamic resources [online]. Available from URL: http://www.boomer.org/pkin [Accessed 2005 Apr 13]
Begré S, Von Bardeleben U, Ladewig D, et al. Paroxetine increases steady-state concentrations of (R)-methadone in CYP2D6 extensive but not poor metabolizers. J Clin Psychopharmacol 2002 Apr; 22(2): 211–5
Hemeryck A, de Vriendt CA, Belpaire FM. Metoprolol-paroxetine interaction in human liver microsomes: stereoselective aspects and prediction of the in vivo interaction. Drug Metab Dispos 2001 May; 29(5): 656–63
Brosen K, Hansen JG, Nielsen KK, et al. Inhibition by paroxetine of desipramine metabolism in extensive but not in poor metabolizers of sparteine. Eur J Clin Pharmacol 1993; 44(4): 349–55
Alderman J, Preskorn SH, Greenblatt DJ, et al. Desipramine pharmacokinetics when coadministered with paroxetine or sertraline in extensive metabolizers. J Clin Psychopharmacol 1997; 17(4): 284–91
Sindrup SH, Brosen K, Gram LF, et al. The relationship between paroxetine and sparteine oxidation polymorphism. Clin Pharmacol Ther 1992 Mar; 51(3): 278–87
Sindrup SH, Brosen K, Gram LF. Pharmacokinetics of the selective serotonin reuptake inhibitor paroxetine: non-linearity and relation to sparteine oxidation polymorphism. Clin Pharmacol Ther 1992 Mar; 51(3): 288–95
Ortiz de Montellano PR, Reich NO. Inhibition of cytochrome P-450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P-450: structure, mechanism, and biochemistry. New York: Plenum Press, 1986: 280–3
Farré M, Roset PN, Hernández-López C, et al. Repeated administration of MDMA to healthy volunteers [abstract]. Drug Alcohol Depend 2001; 63 Suppl.: S46
Ramamoorthy Y, Yu A, Suh N, et al. Reduced (±)-3,4-methylenedioxymethamphetamine (’ecstasy’) metabolism with cytochrome P450 2D6 inhibitors pharmacogenetic variants in vitro. Biochem Pharmacol 2002; 63: 2111–9
Maurer HH, Bickeboeller-Friedrich J, Kraemer T, et al. Toxicokinetics and analytical toxicology of amphetamine-derived designer drugs (’ecstasy’). Toxicol Lett 2000 Mar 15; 112–113: 133–42
Chen YH, Tukey RH. Protein kinase C modulates regulation of the CYP1A1 gene by the aryl hydrocarbon receptor. J Biol Chem 1996 Oct 18; 271(42): 261–6
Kramer HK, Poblete JC, Azmitia EC. Characterization of the translocation of protein kinase C (PKC) by 3,4-methylene-dioxymethamphetamine (MDMA/ecstasy) in synaptosomes: evidence for a presynaptic localization involving the serotonin transporter (SERT). Neuropsychopharmacology 1998 Oct; 19(4): 265–77
Zevin S, Benowitz NL. Drug interactions with tobacco smoking: an update. Clin Pharmacokinet 1999 Jun; 36(6): 425–38
Gilhooly TC, Daly AK. CYP2D6 deficiency, a factor in ecstasy related deaths? Br J Clin Pharmacol 2002 Jul; 54(1): 69–70
Acknowledgements
This study was supported by Fondo de Investigación Sanitaria, Madrid, Spain (grants FIS 97/1198, FIS 98/0181, FIS 00/0777 and FIS 01/1336), ‘Area Progetto Droga’, Istituto Superiore di Sanità, Rome, Italy, and Generalitat de Catalunya, Barcelona, Spain (GENCAT-CIRIT; grant 2001SGR00407).
The authors thank Marta Pulido, MD, for editing the manuscript and for editorial assistance.
The authors have no conflicts of interest directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Segura, M., Farré, M., Pichini, S. et al. Contribution of Cytochrome P450 2D6 to 3,4-Methylenedioxymethamphetamine Disposition in Humans. Clin Pharmacokinet 44, 649–660 (2005). https://doi.org/10.2165/00003088-200544060-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200544060-00006