Abstract
Objective
To study the dose-response relationship of the pharmacokinetic interaction between diltiazem and tacrolimus in kidney and liver transplant recipients.
Design
Nonrandomised seven-period stepwise pharmacokinetic study.
Patients
Stable kidney (n = 2) and liver (n = 2) transplant recipients maintained on oral tacrolimus twice daily but not taking diltiazem.
Methods
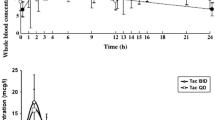
Patients were treated with seven incremental dosages of diltiazem (0 to 180 mg/day) at ≥ 2-weekly intervals. At the end of each interval, 13 blood samples were taken over a 24-hour period to allow determination of morning (AUC12), evening (AUC12–24) and 24-hour (AUC24) areas under the concentration-time curve for tacrolimus, as well as AUC24 for diltiazem and three of its metabolites.
Results
There was considerable interpatient variability in tacrolimus-sparing effect. In the two kidney transplant recipients, an increase in tacrolimus AUC24 occurred following the 20 mg/day dosage of diltiazem (26 and 67%). The maximum increase in tacrolimus AUC24 occurred at the maximum diltiazem dosage used (180 mg/day), when the increase was 48 and 177%. In the two liver transplant recipients, an increase in tacrolimus AUC24 did not occur until a higher diltiazem dosage (60 to 120 mg/day) was given. The increase at the maximum diltiazem dosages used (120 mg/day in one and 180 mg/day in the other) was also lower (18 and 22%) than that exhibited by the kidney transplant recipients. The increase in tacrolimus AUC12 was similar to the increase in AUC12–24 when diltiazem was given in the morning only (dosages ≤60 mg/day). Hence, diltiazem affects blood tacrolimus concentrations for longer than would be predicted from the half-life of diltiazem in plasma.
Conclusions
The mean tacrolimus-sparing effect of diltiazem was similar in magnitude to the cyclosporin-sparing effect previously reported. Whether the lesser tacrolimus-sparing effect with diltiazem seen in the liver transplant recipients was due to functional differences in the transplanted liver is not known, but it was not due to lower plasma diltiazem concentrations. Diltiazem makes a logical tacrolimus-sparing agent because of the potential financial savings and therapeutic benefits. Because of interpatient variability, the sparing effect should be demonstrated in each patient and not merely assumed.




Similar content being viewed by others
Notes
Use of tradenames is for product identification only and does not imply endorsement.
References
Venkataramanan R, Swaminathan A, Prasad T, et al. Clinical pharmacokinetics of tacrolimus. Clin Pharmacokinet 1995; 29: 404–30
Kelly PA, Burckart GJ, Venkataramanan R. Tacrolimus: a new immunosuppressive agent. Am J Health Syst Pharm 1995; 52: 1521–35
Sattler M, Guengerich FP, Yun CH, et al. Cytochrome P-450 3A enzymes are responsible for biotransformation of FK506 and rapamycin in man and rat. Drug Metab Dispos 1992; 20: 753–61
Jain AB, Venkataraman R, Cadoff E, et al. Effect of hepatic dysfunction and T tube clamping on FK 506 pharmacokinetics and trough concentrations. Transplant Proc 1990; 22: 57–9
Spencer CM, Goa KL, Gillis JC. Tacrolimus: an update of its pharmacology and clinical efficacy in the management of organ transplantation. Drugs 1997; 54: 925–75
Trull AK, Tan KKC, Uttridge J, et al. Cyclosporin absorption from microemulsion formulation in liver transplant recipient [letter]. Lancet 1993; 341: 433
Levy G, Rochon J, Freeman D, et al. Cyclosporine Neoral in liver transplant recipients. Transplant Proc 1994; 5: 2949–52
Friman S, Backman L. A new microemulsion formulation of cyclosporin. Pharmacokinetic and clinical features. Clin Pharmacokinet 1996; 3: 181–93
Sokol RJ, Johnson KE, Karrer FM, et al. Improvement of cyclosporin absorption in children after liver transplantation by means of water soluble vitamin E. Lancet 1991; 338: 212–5
Floren LC, Bekersky I, Benet LZ, et al. Tacrolimus oral bio-availability doubles with coadministration of ketoconazole. Clin Pharmacol Ther 1997; 62: 41–9
Christians U, Schmidt G, Bader A, et al. Identification of drugs inhibiting the in vitro metabolism of tacrolimus by human liver microsomes. Br J Clin Pharmacol 1996; 41: 187–90
Teperman L, Turgut S, Negron C, et al. Diltiazem is a safe drug in transplant patients on Prograf and does not affect Prograf levels [AASLD abstract 214]. Hepatology 1996: 180A
Katari SR, Magnone M, Shapiro R, et al. Clinical features of acute reversible tacrolimus (FK 506) nephrotoxicity in kidney transplant recipients. Clin Transplant 1997; 11: 237–42
Regazzi MB, Iacona I, Alessiani M, et al. Interaction between FK 506 and diltiazem in an animal model. Transplant Proc 1996; 28: 1017–8
Seifeldin RA, Marcos-Alvarez A, Gordon FD, et al. Nifedipine interaction with tacrolimus in liver transplant recipients. Ann Pharmacother 1997; 31: 571–5
Pirsch JD, Miller J, Deierhoi MH, et al. A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplant. Transplantation 1997; 63: 977–83
Jones TE. Survey of cyclosporin-sparing agent use in Australasian transplant centres. Aust N Z J Med 1996; 26: 772–6
Chrysostomou A, Walker RG, Russ GR, et al. Diltiazem in renal allograft recipients receiving cyclosporine. Transplantation 1993; 55(2): 300–4
Jones TE, Morris RG, Mathew TH. Diltiazem-cyclosporin pharmacokinetic interaction: dose-response relationship. Br J Clin Pharmacol 1997; 44: 499–504
Morris RG, Saccoia NC, Jones TE. Modified liquid Chromatographic assay for diltiazem and metabolites in human plasma. J Liquid Chromatgr Relat Technol 1996; 19(15): 2385–94
Cogill JL, Taylor PJ, Westley IS, et al. Evaluation of the tacrolimus microparticle enzyme immunoassay (MEIA II) in liver and renal transplant recipients. Clin Chem 1998; 44(9): 1942–6
Morris RG, Jones TE, Mathew TH. Diltiazem disposition and metabolism in renal transplant recipients. Ther Drug Monit 1998; 20: 365–70
Yeung PKF, Prescott C, Haddad C, et al. Pharmacokinetics and metabolism of diltiazem in healthy males and females following a single oral dose. Eur J Drug Metab Pharmacokinet 1993; 18: 199–206
Chen YL, Le Vraux V, Leneveu A, et al. Acute-phase response, interleukin-6, and alteration of cyclosporine pharmacokinetics. Clin Pharmacol Ther 1994; 55: 649–60
Monaco AP, Burke JF, Ferguson RM, et al. Current thinking on chronic renal allograft rejection: issues, concerns and recommendations from a 1997 roundtable discussion. Am J Kidney Dis 1997; 33: 150–60
Didlake RH, Dreyfus K, Kerman RH, et al. Patient noncompliance: a major cause of late graft failure in cyclosporine treated renal transplants. Transplant Proc 1988; 20(3 Suppl. 3): 63–9
Kiley DJ, Lam CS, Pollak R. A study of treatment compliance following kidney transplantation. Transplantation 1993; 55(1): 51–6
Acknowledgements
We are indebted to Associate Professor T.H. Mathew, Mr R. Padbury and Ms L. John for assistance with recruiting patients, and the many members of nursing staff at The Queen Elizabeth Hospital who assisted with blood sampling. We also thank the patients, without whose patience and forbearance the study would not have been possible.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jones, T.E., Morris, R.G. Pharmacokinetic Interaction Between Tacrolimus and Diltiazem. Clin Pharmacokinet 41, 381–388 (2002). https://doi.org/10.2165/00003088-200241050-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200241050-00005