Summary
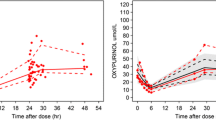
This analysis illustrates the importance of compliance in understanding intrapatient variation in plasma drug concentrations during 2 weeks of repeated (4 times daily) administration. Plasma concentration data are presented from 4 illustrative patients enrolled in a dose-ranging randomised clinical trial comparing diltiazem with placebo for the prevention of painful vaso-occlusive crises in sickle cell disease. Nonlinear regression was used to fit a 1-compartment model (using 1 elimination constant for the first dose and another for subsequent doses) to the observed diltiazem concentration curves for individual patients, taking into account the time of administration of each dose. Actual dosage intervals were obtained from an electronic device (the Medication Event Monitoring System®). The parameters estimated from fitting the actual compliance curves were then used to predict the curves obtained if compliance had been as prescribed (perfect compliance curves). Comparison of the actual and perfect compliance curves shows that within-patient variation in plasma diltiazem concentrations over time can only be understood when the timing of drug administration is included in the analysis. We conclude that dynamic compliance data are important in those situations where close monitoring of treatment is required and may be important in population pharmacokinetic modelling when limited data are available from each patient.
Similar content being viewed by others
References
Boyd RA, Chin SK, Don-Pedro O, Williams RL, Giacomini KH, et al. The pharmacokinetics and pharmacodynamics of diltiazem and its metabolites in healthy adults after a single oral dose. Clinical Pharmacology and Therapeutics 46: 408–419, 1989
Höglund P, Nilsson LG. Pharmacokinetics of diltiazem and its metabolites after repeated multiple-dose treatments in healthy volunteers. Therapeutic Drug Monitoring 11: 543–550, 1989a
Höglund P, Nilsson LG. Pharmacokinetics of diltiazem and its metabolites after repeated single dosing in healthy volunteers. Therapeutic Drug Monitoring 11: 551–557, 1989b
Höglund P, Nilsson LG. Pharmacokinetics of diltiazem and its metabolites after single and multiple dosing in healthy volunteers. Therapeutic Drug Monitoring 11: 5580566, 1989c
Lennert L, Sheiner L, Blaschke T. Improving drug dosing in hospitalized patients: automated modelling of pharmacokinetics for individualization of drug dosage regimens. Computer Methods and Programs in Biomedicine 30: 169–176, 1989
Ludden TM. Population pharmacokinetics. Journal of Clinical Pharmacology 28: 1059–1063, 1988
Pullar T, Kumar S, Tindall H, Feely M. Time to stop counting the tablets? Clinical Pharmacology and Therapeutics 46: 163–168, 1989
Ralston ML, Jennrich RI, Sampson PF, Uno Fk. Fitting pharmacokinetic models with BMDPAR. Technical report no. 58. BMDP Statistical Software 1979. University of California, Los Angeles, 1979
Rudd P, Byyny RL, Zachary V, Loverde ME, Titus C, et al. The natural history of medication compliance in a drug trial: limitations of pill counts. Clinical Pharmacology and Therapeutics 46: 169–176, 1989
Rudd P, Marshall G, Taylor CB, Agras WS. Medication monitor/dispenser for pharmacological and compliance research. Clinical Pharmacology and Therapeutics 29: 178, 1981
Smith MS, Verghese CP, Shand DG, Pritchett EL. Pharmacokinetic and pharmacodynamic effects of diltiazem. American Journal of Cardiology 51: 1369–1374, 1983
Weintraub M. Capricious compliance and intelligent noncompliance. In Lasagna (Ed.) Patient compliance, Vol. 10, pp. 39–47, Louis Futura Publishing Company, Mount Kisco, New York, 1976
Weintraub M. Compliance data: the next level. Hospital Formulary 24: 57–60, 1989
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rubio, A., Cox, C. & Weintraub, M. Prediction of Diltiazem Plasma Concentration Curves From Limited Measurements Using Compliance Data. Clin. Pharmacokinet. 22, 238–246 (1992). https://doi.org/10.2165/00003088-199222030-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-199222030-00006