Abstract
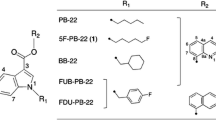
Legally regulated synthetic cannabinoids (SCs) are continuously being created by making minor positional modifications to pre-existing analogs; thus, compounds with minor structural differences must be isolated and identified accurately. For iodo-benzoylindole derivatives of SCs, only specific isomers are currently the target of legal control, and it is necessary to establish an analytical method for accurately identifying positional isomers. In this study, we synthesized a series of 57 designer drugs and developed a screening method for identifying halogen positional isomers on the phenyl ring of benzoylindole derivative SCs in serum. Analytical methods using the Discovery F5 pentafluorophenyl column gave the best selectivity and retention of the positional isomer analytes. Some of the meta and para iodo-substituted SCs were eluted at similar retention times and were difficult to separate by liquid chromatography (LC). However, they were identified via the relative abundance of the two product ions in the collision-induced dissociation reaction using LC-hybrid quadrupole/orbitrap high-resolution mass spectrometry. Our synthesized halogen-substituted positional isomer SC library and method for differentiating positional isomers of halogenated benzoylindole SC derivatives could provide an indispensable analysis tool for identifying illegal drugs in serum of drug users.
Similar content being viewed by others
References
National Institute on Drug Abuse, Synthetic Cannabinoids (K2/Spice) DrugFacts, 2020, https://www.drugabuse.gov/publications/drugfacts/synthetic-cannabinoids-k2spice.
A. C. Howlett, F. Barth, T. I. Bonner, G. Cabral, P. Casellas, W. A. Devane, C. C. Felder, M. Herkenham, K. Mackie, B. R. Martin, R. Mechoulam, and R. G. Pertwee, Pharmacol. Rev., 2002, 54, 161.
R. Kikura-Hanajiri, N. U. Kawamura, and Y. Goda, Drug Test. Anal., 2014, 6, 832.
D. N. Harris, S. Hokanson, V. Miller, and G. P. Jackson, Int. J. Mass Spectrom., 2014, 368, 23.
M. Kusano, K. Zaitsu, H. Nakayama, J. Nakajima, K. Hisatsune, T. Moriyasu, S. Matsuta, M. Katagi, H. Tsuchihashi, and A. Ishii, J. Mass Spectrom., 2015, 50, 586.
S. Borth, W. Hansel, P. Rosner, and T. Junge, J. Mass Spectrom., 2000, 35, 705.
S. Borth, W. Hansel, P. Rosner, and T. Junge, Forensic Sci. Int., 2000, 114, 139.
F. Westphal, P. Rosner, and T. Junge, Forensic Sci. Int., 2010, 194, 53.
T. Murakami, Y. Iwamuro, R. Ishimaru, S. Chinaka, N. Sugimura, and N. Takayama, J. Mass Spectrom., 2016, 51, 1016.
K. M. Abdel-Hay, J. De Ruiter, F. Smith, A. S. Alsegiani, A. Thaxton-Weissenfluh, and C. R. Clark, J. Pharm. Biomed. Anal., 2016, 125, 360.
T. Murakami, Y. Iwamuro, R. Ishimaru, S. Chinaka, N. Takayama, and H. Hasegawa, Forensic Toxicol., 2018, 36, 351.
T. Chikumoto, R. Furukawa, E. Kohyama, K. Suenami, H. Nagai, H. Tada, H. Kawashima, N. Kadomura, M. Soda, K. Kitaichi, and T. Ito, Forensic Toxicol., 2019, 37, 113.
M. N. Eckberg, L. E. Arroyo-Mora, D. R. Stoll, and A. P. DeCaprio, J. Anal. Toxicol., 2019, 43, 170.
H. D. A. Makriyannis, 2001, WO patent 200128557.
P. Jankovics, A. Varadi, L. Tolgyesi, S. Lohner, J. Nemeth-Palotas, and J. Balla, Forensic Sci. Int., 2012, 214, 27.
K. Araki, K. Makino, H. Tabata, H. Nakayama, K. Zaitsu, T. Oshitari, H. Natsugari, and H. Takahashi, Heterocycles, 2018, 96, 910.
K. Araki, H. Tabata, K. Makino, R. Ujiie, K. Sezaki, H. Nakayama, T. Oshitari, H. Natsugari, and H. Takahashi, Heterocycles, 2019, 98, 1423.
M. S. Castaneto, A. Wohlfarth, N. A. Desrosiers, R. L. Hartman, D. A. Gorelick, and M. A. Huestis, Drug Metab. Rev., 2015, 47, 124.
S. Beuck, T. Schwabe, S. Grimme, N. Schlorer, M. Kamber, W. Schanzer, and M. Thevis, J. Am. Soc. Mass Spectrom., 2009, 20, 2034.
S. J. Blanksby and G. B. Ellison, Acc. Chem. Res., 2003, 36, 255.
T. Chikumoto, N. Kadomura, T. Matsuhisa, H. Kawashima, E. Kohyama, H. Nagai, M. Soda, K. Kitaichi, and T. Ito, Forensic Chem., 2019, 13, Article 100157.
M. Hermanns-Clausen, S. Kneisel, M. Hutter, B. Szabo, and V. Auwarter, Drug Test. Anal., 2013, 5, 790.
K. G. Shanks, T. Dahn, and A. R. Terrell, J. Anal. Toxicol., 2012, 36, 145.
T. Saito, A. Namera, N. Miura, S. Ohta, S. Miyazaki, M. Osawa, and S. Inokuchi, Forensic Toxicol., 2013, 31, 333.
A. L. Patton, K. C. Chimalakonda, C. L. Moran, K. R. McCain, A. Radominska-Pandya, L. P. James, C. Kokes, and J. H. Moran, J. Forensic Sci., 2013, 58, 1676.
G. Behonick, K. G. Shanks, D. J. Firchau, G. Mathur, C. F. Lynch, M. Nashelsky, D. J. Jaskierny, and C. Meroueh, J. Anal. Toxicol., 2014, 38, 559.
S. Odoardi, M. Fisichella, F. S. Romolo, and S. Strano-Rossi, J. Chromatogr. B, 2015, 1000, 57.
F. Vaiano, F. P. Busardo, D. Palumbo, C. Kyriakou, A. Fioravanti, V. Catalani, F. Mari, and E. Bertol, J. Pharm. Biomed. Anal., 2016, 129, 441.
Acknowledgments
This project was funded by the Private University Research Branding Project of Japan's Ministry of Education, Culture, Sports, Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Fukuuchi, T., Moriya, Ss., Sugiyama, T. et al. Differentiation of Positional Isomers of Halogenated Benzoylindole Synthetic Cannabinoid Derivatives in Serum by Hybrid Quadrupole/Orbitrap Mass Spectrometry. ANAL. SCI. 37, 329–335 (2021). https://doi.org/10.2116/analsci.20P252
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2116/analsci.20P252