Abstract
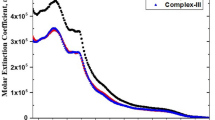
Four useful polypyridine iridium(III) complexes in the form of [IrCl2L2]+ were prepared and their spectroscopic and electrochemical properties as well as X-ray crystallography were investigated. The ligands used were L = 2,2’-bipyridine, 4,4’-dimethyl-2,2’-bipyridine, 4,4’-diphenyl-2,2’-bipyridine, 1,10-phenanthroline, 4,7-diphenyl-1,10-phenanthroline, and 2,2’-biquinoline. Synthetic methods were developed by a sequential ligand-replacement, which occurred in the reaction vessel using a microwave oven. All complexes showed that LUMOs are based on the π-system contribution of the polypyridine ligand for [IrCl2(bpy)2]+, [IrCl2(dmbpy)2]+, [IrCl2(dpbpy)2]+, [IrCl2(phen)2]+, [IrCl2(dpphen)2]+ and [IrCl2(bqn)2]+. The HOMOs are also localized on the polypyridine ligand in the iridium complexes. It was found that [IrCl2L2]+ emits intense phosphorescence at room temperature. In particular, the use of dpbpy as ancillary ligands extends the lifetime (660 ns) of the 3(π-π*) excited states of Ir(III) polypyridine complexes. The complex [IrCl2(bqn)2]+ with electron acceptor substituents shows a large red-shift to 622 nm. It is noticed that iridium polypyridine complexes show intense emissions at various colors, such as yellow for [IrCl2(dmbpy)2]+ and red for [IrCl2(bqn)2]+, which can be applied to photosensitizers. The spectroscopic and electrochemical details are also reported herein.
Similar content being viewed by others
References
L. M. Volgler and K. J. Brewer, Inorg. Chem., 1996, 35, 818.
J. A. Treadway, B. Loeb, R. Lopez, P. A. Anderson, F. R. Keene, and T. J. Meyer, Inorg. Chem., 1996, 35, 2242.
S. Lamansky, P. Djurovich, D. Murphy, F. Abdel-Razzaq, R. Kwong, I. Tsyba, M. Bortz, B. Mui, R. Bau, and M. E. Thompson, Inorg. Chem., 2001, 40, 1704.
S. Lamansky, P. Djurovich, D. Murphy, F. Abdel-Razzaq, H. E. Lee, C. Adachi, P. E. Burrows, S. R. Forrest, and M. E. Thompson, J. Am. Chem. Soc., 2001, 123, 4304.
F. Neve, A. Crispini, S. Campagna, and S. Serroni, Inorg. Chem., 1999, 38, 2250.
N. Yoshikawa and T. Matsumura-Inoue, Anal. Sci., 2003, 19, 761.
A. Dovletoglou, S. A. Adeyemi, and T. J. Meyer, Inorg. Chem., 1996, 35, 4120.
S. M. Zakeeruddin, M. K. Nazeeruddin, P. Pechy, F. P. Rotzinger, R. Humphry-Baker, K. Kalyanasundaram, and M. Gratzel, Inorg. Chem., 1997, 36, 5937.
A. B. P. Lever, Inorg. Chem., 1990, 29, 1271.
B. Chiswell and S. E. J. Livingstone, Inorg. Nucl. Chem., 1964, 26, 47
N. Yoshikawa, Y. Masuda, and T. Matsumura-Inoue, Chem. Lett., 2000, 1206.
S. S. Fielder, M. C. Osborne, A. B. P. Lever, and W. J. Pietro, J. Am. Chem. Soc., 1995, 117, 6990.
J. V. Casper and T. J. Meyer, Inorg. Chem., 1983, 22, 2444.
P. A. Anderson, L. F. Anderson, M. Furue, P. C. Junk, F. R. Keene, B. T. Patterson, and B. D. Yeomans, Inorg. Chem., 2000, 39, 2721.
N. Yoshikawa, J. Sakamoto, N. Kanehisa, Y. Kai, T. Matsumura-Inoue, H. Takashima, and K. Tsukahara, Acta Crystallogr., 2003, E59, m155.
N. Yoshikawa, J. Sakamoto, N. Kanehisa, Y. Kai, K. Matsumura, H. Takashima, and K. Tsukahara, Acta Crystallogr., 2003, E59, m551.
N. Yoshikawa, J. Sakamoto, N. Kanehisa, Y. Kai, T. Matsumura-Inoue, H. Takashima, and K. Tsukahara, Acta Crystallogr., 2003, E59, m972.
I. M. Dixon, J. P. Collin, J. P. Sauvage, L. Flamigni, S. Encinas, and F. Barigelletti, Chem. Soc. Rev., 2000, 29, 385.
M. T. Indelli, C. A. Bignozzi, and F. Scandola, Inorg. Chem., 1998, 37, 6084.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshikawa, N., Sakamoto, J., Matsumura-Inoue, T. et al. Electrochemical and Phosphorescent Properties of New Ir(III) Complexes Coordinated by Various Bipyridine Derivatives. ANAL. SCI. 20, 711–716 (2004). https://doi.org/10.2116/analsci.20.711
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2116/analsci.20.711