
Taking it up a notch: a promising immunotherapy against small cell lung cancer
In the past two decades, there has been a major effort to develop strategies harnessing the immune system to inhibit the growth of tumors. These efforts, including therapies targeting programmed cell death protein 1 and programmed death ligand 1 (PD-1/PD-L1), have led to substantial and often durable responses in many settings, including in subsets of non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) patients (1). However, the overall decrease in mortality from these therapies remains limited, especially for SCLC, highlighting the need for new strategies. A recent Phase 1 study with Tarlatamab, a first-in-class delta-like ligand 3 (DLL3)-targeted bispecific T cell engager (2), provides some encouraging news for patients with SCLC.
SCLC is a fatal neuroendocrine cancer that accounts for ~15% of all lung cancer cases and causes over 200,000 deaths worldwide annually (3). SCLC most often develops in the lungs of heavy smokers. From 2000 to 2015, the observed decrease in SCLC deaths in the US was a result only of decreased incidence (1). Unfortunately, the number of SCLC-related deaths continues to rise worldwide due to a continuous increase in the number of smokers. A major clinical challenge is that a large majority of patients with SCLC have disseminated disease at the time of first diagnosis; these patients have a 5-year survival of <1% [reviewed in (4)]. Even patients with localized disease at diagnosis have dismal survival rates. A second related challenge is that almost all patients treated with standard-of-care chemotherapy and radiation therapy relapse rapidly after a good initial response. There is a current unmet need to understand the mechanisms of tumor development, cell behavior, and immune escape to eventually develop effective therapeutic approaches in SCLC, including immunotherapy approaches that could be combined with standard-of-care chemoradiation therapy.
SCLC tumors have a high tumor mutation burden, and activation of T cells would be expected to result in strong anti-tumor effects in SCLC. However, immune checkpoint blockade yields a median increased survival of only a few months [reviewed in (5)]. A number of factors may explain these observations, including low expression of MHC class I on the surface of neuroendocrine SCLC cells, which limits the ability of cytotoxic T cells to attack SCLC cells (6). Thus, while immune checkpoint inhibitors have become standard for SCLC treatment in the clinic, their limited effectiveness underscores the need for new immunotherapies that more effectively harness immune cells in SCLC. In particular, T-cell-based therapies that do not require expression of major histocompatibility complex (MHC) class I on the surface of SCLC cancer cells, including chimeric antigen receptor (CAR) T cells, antibody-drug conjugates (ADCs), and bispecific T-cell engagers (BiTEs), may alleviate some of the issues with conventional immune checkpoint blockade.
A key challenge in the field is to identify molecules on the surface of SCLC cells that could be chosen as targets for CAR T cells, ADCs, or BiTEs. DLL3 is an atypical ligand in the Notch signaling pathway involved in embryonic development, and it is normally exclusively expressed in the Golgi of cells, where it is thought to inhibit Notch signaling by interacting in cis with NOTCH receptors (7). Importantly, Saunders and colleagues showed that, unlike normal cells in the body, DLL3 is specifically present at the surface of SCLC cells (8). The DLL3 gene is a target of the ASCL1 transcription factor (9), and it is expressed in a large majority of SCLC cases [reviewed in (10)]. DLL3’s role in the biology of SCLC is still poorly characterized, but DLL3 may contribute to Notch signaling (11) and to SCLC cell migration (12). Targeting DLL3-expressing SCLC cells using an ADC was shown to eradicate SCLC in pre-clinical models (8). Unfortunately, in the clinic, the Rovalpituzumab tesirine ADC (Rova-T) has been unsuccessful due to a combination of lack of efficacy and toxic side effects of the pyrrolobenzodiazepine (PDB) payload [(13) and references herein]. While it is likely that ADCs targeting DLL3 with other linkers or payloads would show some efficacy in the clinic with fewer side effects, a number of groups have turned to other approaches including BiTEs (14-16), such as the recent publication of a clinical Phase 1 study with Tarlatamab (2). Tarlatamab (AMG 757) is a half-life extended BiTE bridging DLL3 on cancer cells and CD3 on T cells, thus enabling T-cell-mediated lysis of DLL3-expressing SCLC tumors (Figure 1A). In this safety and tolerability study, 107 heavily pretreated SCLC patients were given Tarlatamab intravenously in a dose escalation regimen (2). The most common adverse effects identified were transient cytokine release syndrome (in ~50% of patients), pyrexia (~40%), and constipation (~30%), which were all resolved with appropriate care. Grade 3 neutropenia was observed in ~10% of patients, which is likely an indirect effect of treatment since DLL3 is not thought to be expressed by neutrophils. Thus, the tolerability for Tarlatamab seems acceptable in patients with SCLC, even in those patients who were heavily treated before the study. This study also shows some promising antitumor activity with an objective response rate of ~20%, including 2 complete and 23 partial responses, an overall survival of 13.2 months, and a median progression-free survival of 3.7 months. Overall, in this Phase 1 study, Tarlatamab compares favorably to Rova-T, and these clinical observations provide additional support for DLL3 as a promising target in SCLC.
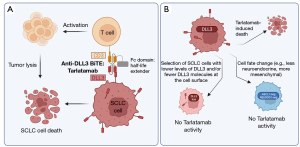
Data from this Phase 1 study raise a number of questions about the best path forward for Tarlatamab in the clinic and the best use of DLL3 as a target. As would be expected, an exploratory analysis from the current data suggests that higher DLL3 expression on the surface of SCLC cells correlates with increased clinical benefit (2). Because DLL3 is a direct target of ASCL1 and is more highly expressed in SCLC cells with strong neuroendocrine features, selection of patients whose tumors fit these criteria (i.e., DLL3-high, ASCL1-high, neuroendocrine-high) may help ensure better antitumor responses with Tarlatamab. The patients most likely to respond to treatment could be identified from biopsy samples and from circulating tumor cells (CTCs) [see for example (17)]. Fast and strong responses may be important in this type of approach because it is unclear whether DLL3 plays a critical role in the biology of SCLC, and its expression (or its specific expression on the cell surface) may not be required for the long-term growth of SCLC tumors. If this is the case, there may be rapid selection for DLL3-low cells, which may not respond as well to Tarlatamab [especially since DLL3 levels are already low on SCLC cells compared to other cell surface targets (18)] (Figure 1B). This also raises questions of how Tarlatamab can be used in combination with other therapies and whether this molecule is better suited as a first-line therapy or later in the treatment strategy. In particular, it is possible that trials conducted in heavily pretreated patients may underestimate the true therapeutic potential of Tarlatamab: indeed, increasing evidence indicates that SCLC tumors respond to chemotherapy by changing their overall differentiation state, including with a shift towards less neuroendocrine differentiation [e.g., a more mesenchymal state (19)] (Figure 1B). Because DLL3 expression tends to be lower in less neuroendocrine SCLC cells, this shift may be accompanied by a decrease in the number of DLL3 molecules at the cell surface and decreased efficacy of Tarlatamab. On the other hand, chemotherapy (or radiation therapy) may help generate an inflamed microenvironment that may recruit immune cells such as T cells and thus potentiate the efficacy of this BiTE. Similarly, DLL3 levels are very low in tumors classified as “SCLC-I”, an inflamed subtype of SCLC with low neuroendocrine differentiation but greater response to immune checkpoint inhibitors that activate cytotoxic T cells (20). Thus, it is unclear which subtype of SCLC tumors would respond best to a combination of Tarlatamab and immune checkpoint inhibitors such as anti-PD-L1 therapies, as DLL3 expression seems to be highest when T cells are less present and/or less active in the tumor microenvironment. These questions may be resolved in future clinical trials with more patients and a biomarker analysis of their tumors before and after treatment.
The number of DLL3 molecules on the surface of SCLC cells is low [<10,000, see (18)], which is a limitation for any strategy targeting this molecule. One way to increase the efficacy of Tarlatamab would be to gain a better understanding of the mechanisms that lead to the expression of DLL3 on the surface of SCLC cells (while it is expressed only in the Golgi of normal cells). Improving the affinity of DLL3 binders may also help boost the efficacy of Tarlatamab. These strategies may be especially important in cases where SCLC cells lose their neuroendocrine features and display even fewer DLL3 molecules on their cell surface.
CAR T cells are being developed against DLL3 in parallel to the Tarlatamab BiTE. Recent pre-clinical studies [e.g., (21-23)] suggest good efficacy and safety. It is unclear whether anti-DLL3 CAR T cells will be a better therapeutic tool than BiTEs such as Tarlatamab in patients with SCLC. This question will need to be resolved with additional clinical trials.
Lastly, DLL3 is expressed on the surface of not just SCLC but also a large number of other neuroendocrine tumors, including neuroendocrine prostate cancer, large-cell neuroendocrine carcinoma of the lung, gastroenteropancreatic neuroendocrine neoplasms, Merkel cell cancer (a neuroendocrine form of skin cancer), and neuroblastoma [see (24) and discussed in (25)]. Many of these neuroendocrine tumors also lack therapeutic options, and molecules such as Tarlatamab may thus be useful in the treatment of neuroendocrine tumors beyond SCLC.
In conclusion, this study opens the door to a promising anti-DLL3 BiTE therapy against SCLC and provides further support for DLL3 as a promising target in SCLC and other neuroendocrine tumors. As with other targeted therapies, it may be important to understand the biology of the target (including the mechanisms that control its presence on the cell surface and its biological role in SCLC cells) as well as the biology of the tumor (including intra- and inter-cellular heterogeneity as well as plasticity). Ultimately, it is likely that a subset of patients with SCLC will benefit from Tarlatamab, and the major goal of the next couple of years will be to identify these patients.
Acknowledgments
The authors thank Dr. Julie Ko for her feedback on the manuscript and apologize to investigators in the field whose work could not be cited due to space limitations.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Lung Cancer Research. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-230/coif). Research reported in this publication was supported by the Ludwig Institute for Cancer Research (JS) and the NIH (grants CA217450 and CA231997 to JS). JS has equity in, and is an advisor for, DISCO Pharmaceuticals. AA has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Howlader N, Forjaz G, Mooradian MJ, et al. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N Engl J Med 2020;383:640-9. [Crossref] [PubMed]
- Paz-Ares L, Champiat S, Lai WV, et al. Tarlatamab, a First-In-Class DLL3-Targeted Bispecific T-Cell Engager, in Recurrent Small Cell Lung Cancer: An Open-Label, Phase I Study. J Clin Oncol 2023; Epub ahead of print. [Crossref] [PubMed]
- Rudin CM, Brambilla E, Faivre-Finn C, et al. Small-cell lung cancer. Nat Rev Dis Primers 2021;7:3. [Crossref] [PubMed]
- Ko J, Winslow MM, Sage J. Mechanisms of small cell lung cancer metastasis. EMBO Mol Med 2021;13:e13122. [Crossref] [PubMed]
- Barrows ED, Blackburn MJ, Liu SV. Evolving role of immunotherapy in small cell lung cancer. Semin Cancer Biol 2022;86:868-74. [Crossref] [PubMed]
- Burr ML, Sparbier CE, Chan KL, et al. An Evolutionarily Conserved Function of Polycomb Silences the MHC Class I Antigen Presentation Pathway and Enables Immune Evasion in Cancer. Cancer Cell 2019;36:385-401.e8. [Crossref] [PubMed]
- Geffers I, Serth K, Chapman G, et al. Divergent functions and distinct localization of the Notch ligands DLL1 and DLL3 in vivo. J Cell Biol 2007;178:465-76. [Crossref] [PubMed]
- Saunders LR, Bankovich AJ, Anderson WC, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med 2015;7:302ra136. [Crossref] [PubMed]
- Augustyn A, Borromeo M, Wang T, et al. ASCL1 is a lineage oncogene providing therapeutic targets for high-grade neuroendocrine lung cancers. Proc Natl Acad Sci U S A 2014;111:14788-93. [Crossref] [PubMed]
- Zhang H, Yang Y, Li X, et al. Targeting the Notch signaling pathway and the Notch ligand, DLL3, in small cell lung cancer. Biomed Pharmacother 2023;159:114248. [Crossref] [PubMed]
- Kim JW, Ko JH, Sage J. DLL3 regulates Notch signaling in small cell lung cancer. iScience 2022;25:105603. [Crossref] [PubMed]
- Furuta M, Kikuchi H, Shoji T, et al. DLL3 regulates the migration and invasion of small cell lung cancer by modulating Snail. Cancer Sci 2019;110:1599-608. [Crossref] [PubMed]
- Blackhall F, Jao K, Greillier L, et al. Efficacy and Safety of Rovalpituzumab Tesirine Compared With Topotecan as Second-Line Therapy in DLL3-High SCLC: Results From the Phase 3 TAHOE Study. J Thorac Oncol 2021;16:1547-58. [Crossref] [PubMed]
- Giffin MJ, Cooke K, Lobenhofer EK, et al. AMG 757, a Half-Life Extended, DLL3-Targeted Bispecific T-Cell Engager, Shows High Potency and Sensitivity in Preclinical Models of Small-Cell Lung Cancer. Clin Cancer Res 2021;27:1526-37. [Crossref] [PubMed]
- Hipp S, Voynov V, Drobits-Handl B, et al. A Bispecific DLL3/CD3 IgG-Like T-Cell Engaging Antibody Induces Antitumor Responses in Small Cell Lung Cancer. Clin Cancer Res 2020;26:5258-68. [Crossref] [PubMed]
- Tully KM, Tendler S, Carter LM, et al. Radioimmunotherapy Targeting Delta-like Ligand 3 in Small Cell Lung Cancer Exhibits Antitumor Efficacy with Low Toxicity. Clin Cancer Res 2022;28:1391-401. [Crossref] [PubMed]
- Chemi F, Pearce SP, Clipson A, et al. cfDNA methylome profiling for detection and subtyping of small cell lung cancers. Nat Cancer 2022;3:1260-70. [Crossref] [PubMed]
- Sharma SK, Pourat J, Abdel-Atti D, et al. Noninvasive Interrogation of DLL3 Expression in Metastatic Small Cell Lung Cancer. Cancer Res 2017;77:3931-41. [Crossref] [PubMed]
- Stewart CA, Gay CM, Xi Y, et al. Single-cell analyses reveal increased intratumoral heterogeneity after the onset of therapy resistance in small-cell lung cancer. Nat Cancer 2020;1:423-36. [Crossref] [PubMed]
- Gay CM, Stewart CA, Park EM, et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell 2021;39:346-360.e7. [Crossref] [PubMed]
- Zhang Y, Tacheva-Grigorova SK, Sutton J, et al. Allogeneic CAR T Cells Targeting DLL3 Are Efficacious and Safe in Preclinical Models of Small Cell Lung Cancer. Clin Cancer Res 2023;29:971-85. [Crossref] [PubMed]
- Jaspers JE, Khan JF, Godfrey WD, et al. IL-18-secreting CAR T cells targeting DLL3 are highly effective in small cell lung cancer models. J Clin Invest 2023;e166028. [Crossref] [PubMed]
- Chen X, Amar N, Zhu Y, et al. Combined DLL3-targeted bispecific antibody with PD-1 inhibition is efficient to suppress small cell lung cancer growth. J Immunother Cancer 2020;8:e000785. [Crossref] [PubMed]
- Krytska K, Casey CE, Pogoriler J, et al. Evaluation of the DLL3-targeting antibody-drug conjugate rovalpituzumab tesirine in preclinical models of neuroblastoma. Cancer Res Commun 2022;2:616-23. [Crossref] [PubMed]
- Yao J, Bergsland E, Aggarwal R, et al. DLL3 as an Emerging Target for the Treatment of Neuroendocrine Neoplasms. Oncologist 2022;27:940-51. [Crossref] [PubMed]


