
Current literature review on the tumor immune micro-environment, its heterogeneity and future perspectives in treatment of advanced non-small cell lung cancer
Introduction
Lung cancer is one of the most common malignancies causing cancer-related deaths, and non-small cell lung cancer (NSCLC) accounts for 85% of all newly diagnosed lung cancer cases (1,2). Immune checkpoint inhibitors (ICIs) were a major clinical advancement that provided an opportunity to improve the prognosis of patients with NSCLC. Patients who show high programmed death-ligand-1 (PD-L1) expression from tumor cells are more likely to benefit from ICIs (3-5); however, PD-L1 expression does not sufficiently predict ICI efficacy in NSCLC patients. In addition, PD-L1 expression does not fully explain the ICI mode of action, and complex underlying mechanisms likely exist. ICIs neutralize programmed cell death 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA4) pathways that directly and indirectly block the activation of T cells to restore anti-tumor activities of immune cells (6,7), but other non-immune cells are also significantly involved in anti-tumor responses.
In recent studies, the tumor immune microenvironment (TIME) was shown to have a central role in lung cancer development and to affect clinical outcome of patients diagnosed with lung cancer (8,9). The TIME is a collection of tumor and non-tumor cells, with involvement of various cytokines, extracellular matrix (ECM), and vessels. The TIME is dynamic, and involved cells participate in treatment resistance and show complex interactions during malignant progression by activation of inhibitory immune checkpoints. As development of new therapeutic targets to overcome ICI resistance is a priority, understanding the TIME is important. The heterogeneity of TIME can be spatial and temporal and significantly influence efficacy of anti-cancer treatment modalities, especially ICIs. Recently, a series of studies was conducted to target the immunosuppressive component of TIME to overcome resistance to tumor treatment. In this review, important features regarding the TIME and its heterogeneity and importance in practical NSCLC management are discussed. We present the following article in accordance with the Narrative Review reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-633/rc).
Methods
PubMed and PMC were searched from January 1st, 2012 to August 16th, 2022 using the following key words: “NSCLC”, “Tumor microenvironment”, “Immune”, “Metastasis” and “Heterogeneity” (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | 16.08.2022 |
| Databases and other sources searched | PubMed, PMC |
| Search terms used | “non-small cell lung cancer”, “Tumor microenvironment”, “Immune”, “Metastasis” and “Heterogeneity” |
| Timeframe | 01.01.2012 to 16.08.2022 |
| Inclusion and exclusion criteria | Original publications, reviews, clinical trials and abstract were included |
| Selection process | Selection by authors |
Overview of TIME
The TIME is comprised of both tumor cells and nonmalignant cells, including fibroblasts, pericytes, adipocytes, vascular endothelial cells, various immune cells, carcinoma-associated fibroblasts (CAFs), ECM, and blood and lymphatic vessels (10-12) (Figure 1). Secretion of cytokines and growth factors and tissue matrix remodeling by tumor cells are involved in suppression of immune cells in the TIME (10,13). For clinical application, each TIME component should be subgrouped into either pro-immunogenic or immunosuppressive to determine the potential target of clinical intervention.
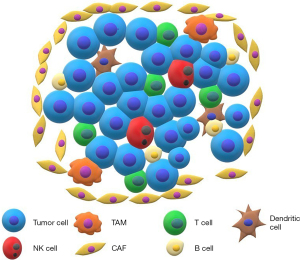
Recognition of tumor-associated antigens (TAAs) is thought to trigger the tumor-immune cell response (14,15). Signals from proliferating tumor cells in the TIME may activate innate cells including natural killer (NK) cells (15). Debris from tumor cells and damage-associated molecular patterns further recruit antigen-presenting cells (APCs), mainly dendritic cells (DCs), into the tumor microenvironment (16). DCs capture tumor antigens; type I DCs initiate CD8 T cell responses (17), while type II DCs are responsible for initiation of CD4 responses (18). The activated cytotoxic T lymphocytes in the TIME are re-stimulated by tumor-resident APCs or major histocompatibility complex (MHC) class I molecules on tumor cells, with subsequent death of tumor cells (19).
However, tumors can progress despite the presence of tumor-antigen-specific CD8+ T-cell populations, largely due to immunosuppressive activities, both from tumor cells and the adjacent environment. Subtypes of cells in the TIME such as tumor cells, regulatory T (Treg) cells, suppressive myeloid cells, CAFs, vascular endothelial cells, and regulatory B cells can be immunosuppressive (20).
Treg cells are a subset of CD4-positive T cells and generally express the transcription factor Forkhead box protein P3 (FoxP3) (21). Treg cells suppress the activity of other immune cell subsets and prevent excessive immune responses to self-and non-self-antigens, contributing to immune homeostasis (22). Furthermore, Treg cells can promote carcinogenesis and cancer progression by inactivating anti-tumor immunity (23). Abundance of Treg cells is generally associated with poor clinical outcome in lung cancer. One meta-analysis that included 1,303 NSCLC patients from 11 studies showed association between increased tumor-infiltrating FoxP3+ Tregs cells and poor overall survival (OS) (24). Another study that included 196 NSCLC and 137 normal samples also showed strong association between tumor-infiltrating Treg-related genes and poor OS (25).
CAFs and the ECM also contribute to development and maintenance of an immunosuppressive microenvironment. CAFs are involved in production of ECM components of the tumor microenvironment (26) and secretion of paracrine ligands that promote tumor growth, vessel formation, and drug resistance (27). In various types of malignancies, CAFs play roles in recruiting Treg cells, myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs), contributing to formation of an immunosuppressive tumor microenvironment (28,29).
MDSCs derive from immature myeloid progenitors and, in general, are categorized into two subpopulations, polymorphonuclear MDSCs (PMN-MDSCs) and monocytic MDSCs (M-MDSCs) (30-32). MDSCs contribute to the control of anti-cancer immune responses and are involved in tumor progression by activating tumor angiogenesis, tumor cell proliferation, and formation of a premetastatic niche (33).
TAMs can have pro- or anti-tumor properties depending on phenotype (M1 vs. M2) (34). TAMs (M2 type) contribute to tumor growth, immunosuppression, and cancer cell invasion. In addition, they play a central role in therapeutic resistance by secreting transforming growth factor beta (TGF-β), chemokine (C-C motif) ligand 18 (CCL18), interleukin-10 (IL-10), matrix metalloproteases, vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF)-B (35). Tables 2,3 list the important immune and non-immune cells, cytokines, chemokines, and other related proteins based on the predominant immunogenic or immunosuppressive characteristics. Table 4 shows the major cells in the TIME and their related molecular markers, role in tumor microenvironment, and association with prognosis of lung cancer.
Table 2
| Predominantly immunogenic | Predominantly immunosuppressive |
|---|---|
| Immune and non-immune cells | |
| Cytotoxic CD8 T cells | Treg cells |
| NK cells | MDSC |
| Dendritic cells (lymphoid related) | CAF |
| Tumor-associated macrophage (M1) | Tumor-associated macrophage (M2) |
NK, natural killer; Treg cell, regulatory T cell; MDSC, myeloid-derived suppressor cell; CAF, cancer-associated fibroblast.
Table 3
| Immunogenic | Role | References | Immuno-suppressive | Role | References |
|---|---|---|---|---|---|
| IL-2 | Potent inducer of cytotoxic T cells and NK cells | (36) | IL-2 | Induces proliferation of regulatory T cells by binding to IL-2 receptor alpha (IL-2Ra) | (37) |
| IL-10 | May stimulate tumor-resident CD8+ T cell by inducing cytotoxicity of CD8+ T cells, resulting in increase in the expression of IFN-γ in CD8+ T cells | (38) | IL-10 | Secreted by numerous tumor cells | (39) |
| Enhances tumor cell survival, proliferation, and metastasis Suppressive effects on other effector immune cells |
|||||
| Mainly secreted by cancer cells, and immune cells including myeloid and lymphoid lineages | |||||
| IL-12 | Production of IL-12 induced by activated APCs and T cells Proven to have an antitumor role in mouse models of lung cancer |
(40,41) | IL-6 | IL-6 reduces the effectiveness of anti-PD-L1 blockade in a mouse model | (42) |
| IL-27 | Potent anti-tumor effects by inhibiting angiogenesis inhibition, granzyme B expression promotion, and proliferation of effective anti-tumor T cells | (43-45) | IL-27 | Restrain immune responses via CD39 expression in Tregs, and increase expression of multiple co-inhibitory receptors and co-inhibitory ligands | (45,46) |
| TNF-α | Produced by immune cells including monocytes Contributes to cytotoxic T cells’ antitumor activity and enhances tumor cytotoxicity by lowering threshold to T cell-derived TNF-α |
(47-49) | TNF-α | Decrease antitumor immune response by activation of a subgroups of immunosuppressive cells including Tregs and regulatory B cells | (50,51) |
| Calreticulin on tumor cells | Endoplasmic reticulum (ER)-resident protein involved in protein folding Supports anti-tumor immune response initiation |
(52) | MMPs | Resident in tumor microenvironment | (53) |
| Involved in matrix disruption, angiogenesis, and cancer cell metastasis | |||||
| Interferon-γ | Interferon-γ induced programmed cell death by activation of JAK-STAT1 signaling pathway in non-small cell lung cancer cell lines Indirectly inhibition of M2-like immunosuppressive tumor-associated macrophages by inhibiting fatty acids synthesis |
(54,55) | LAG | LAG-3 are expressed on activated T cells Regulate the function of T cells to maintain the homeostasis of the immune system Promote immune escape of tumor cells in the tumor microenvironment |
(56) |
| Interferon-γ | Induction of immune checkpoint receptor, PD-L1 and indoleamine in tumor tissue | (57) | |||
| Indoleamine | Promotes immunosuppressive effects by regulating the metabolism of amino acid tryptophan and kynurenine in the tumor microenvironment Induces Treg differentiation |
(58,59) | |||
| Adenosine | Adenosine signaling impedes dendritic cell maturation and inhibit differentiation of effector cell | (60,61) | |||
| PGE2 | Produced by cancerous stromal cells | (62) | |||
| Suppresses immune reactions against tumor cells and involved in tumor immune evasion | |||||
| HIF-1 α | HIF-1α inhibition decreases tumor immunosuppression, and combination with immune checkpoint inhibitors induces tumor regression in a mouse model | (63) | |||
| TGF-β | Induce T reg differentiation Immune suppression in tumor microenvironment |
(39) | |||
| IL-4 | Secreted by basophils, mast cells, and Th2 cells | (64,65) | |||
| Regulation of humoral immune responses | |||||
| IL-4 downstream STAT6-activated signaling in myeloid cells promotes M2 immune-suppressive phenotype, and enhances lung cancer progression |
APCs, antigen presenting cells; NK, natural killer; Treg cell, regulatory T cell; IL, interleukin; TNF-α, tumor necrosis factor alpha; JAK-STAT, janus kinase-signal transducer and activator of transcription; MMP, matrix metalloproteinase; LAG, lymphocyte-activation gene; PD-L1, programmed cell death 1 ligand 1; PGE2, prostaglandin E2; HIF-1, hypoxia-inducible factor; TGF-β, transforming growth factor beta; Th2, type 2 T helper.
Table 4
| Cell population | Key related markers | Role in TIME | Prognostic values in lung cancer | References |
|---|---|---|---|---|
| Lymphoid | ||||
| Cytotoxic CD8+ T cells | CD8+, CD3+ | •Direct cytotoxic effects on cancer cells by secreting granzymes and perforin •Cytotoxic lymphocytes are re-stimulated by tumor-resident APC or MHC class I on tumor cells, kill tumor cells, and spread neoantigen responses that may trigger secondary immune responses |
•In a study of pretreatment biopsy samples acquired from 199 patients with stage IV non-small cell lung cancer, the proportion of CD8(+) T cells between cancer nests and stroma was independently associated with survival •A retrospective study of 33 stage II–IV NSCLC patients showed that the response to immunotherapy was significantly better in the group with high infiltration of CD8+ T cells than the low-infiltration group |
(19,66-68) |
| B cells | CD19+, CD20+ | •B cells have both pro- and anti-tumor roles depending on phenotype •Infiltrating B cells have been shown to act in presentation of antigens to CD4+ T cells, resulting in T cell effector activities •Pro-tumor activity was suggested for IL-10-producing B cells for immunosuppressive features |
•In a study of 196 patients with NSCLC treated with neoadjuvant chemotherapy, increased B cells were associated with improved DFS •Association with B cells and NSCLC prognosis was not concretely demonstrated •Despite results from other cancers, no association between B cell density and response to checkpoint blockade has been shown |
(66,69-74) |
| T regulatory cells | CD3+CD4+Foxp3+ CD35+ | •Prevent excessive immune responses to self- and nonself-antigens to maintain immune homeostasis •Can promote tumor growth by inhibiting anti-tumor responses |
•A meta-analysis showed that high level of FoxP3+ Tregs was significantly associated with unfavorable prognosis in NSCLC | (23,24,66) |
| Resident memory T (TRM) CD8 cells | Components of CD8+ cells, CD103+, CD69, CD49a, and PD-1, Can vary according to tumor site | •Unlike lymphocytes in blood circulation, TRMs reside in peripheral tissues, rapidly respond to hazard signals, and contribute to antitumor surveillance and immunity |
•Increased density of CD103+ and CD8+ lymphocytes in immunotherapy-naive tumors is associated with greatly improved outcomes of immunotherapy | (75-77) |
| Myeloid | ||||
| Dendritic cells |
CD11c+, CD141+, CD83+ | •DCs capture tumor antigen. Type I DCs initiate CD8 T cell responses, while type II DCs are responsible for initiation of CD4 responses | •In a retrospective study of 99 patients with NSCLC, number of mature DCs in tumor specimens was positively associated with patient survival in univariate analysis but not multivariate analysis | (16,18,69,78) |
| MDSCs | PMN-MDSCs: CD11b+CD14−CD15+/CD66b+ | •Categorized into PMN-MDSCs and M-MDSCs | •Poor recurrence-free survival in patients with high PMN-MDSC level in blood, but no correlation between prognosis and PMN-MDSCs from tumor | (30-33,79,80) |
| M-MDSCs: CD11b+CD15−CD14+HLA-DR−/low | •Contribute to the control of antitumor immune responses •Involved in tumor progression by activating tumor angiogenesis, tumor cell proliferation, and formation of a pre-metastatic niche |
•A meta-analysis including NSCLC showed that a high level of pretreatment circulating MDSCs shows a negative influence on survival in most cancers | ||
| TAMs | CD68+, CD163+ | •TAMs (M2 type) contribute to tumor growth, immunosuppression, and cancer cell invasion. Central role in therapeutic resistance by secreting TGF-β, CCL18, IL-10, matrix metalloproteases, VEGF, and PDGF-B | •High density of M2 macrophages was independently predictive of incidence of spread through air spaces (STAS) in stage 0–I lung adenocarcinoma | (35,81-83) |
| •M1 phenotype is pro-inflammatory, secretes TNF-α and nitric oxide to kill tumor cells, and further activates T-cell-mediated immune response | ||||
| NK cells | CD16+, CD56+, CD57+, CD58+ | •Show cytotoxic activity against infected and mutated cells •Express immune cell activation and inhibitory receptors •Secrete cytokines and chemokines such as tumor necrosis factor α, interferon γ, C-C motif chemokine ligand 3, and GM-CSF to interact with other immune cells |
•In a study of 60 squamous cell lung cancer patients, presence of tumor infiltrating natural killer cells (CD57) from surgical specimens was associated with postoperative survival •Significant difference in survival between patients whose tumors had high vs. low natural killer cell counts •Presence of NK cells from tumor samples did not affect the prognosis of patients with NSCLC |
(66,69,82,84-87) |
| Else | ||||
| CAFs | α-SMA, fibroblast-specific protein-1 | •Involved in production of extracellular matrix components of the tumor microenvironment •Secretion of paracrine ligands that promote tumor growth, vessel formation, and drug resistance •Recruit Treg cells, MDSCs, and TAMs |
•Analysis of 517 patients with lung adenocarcinoma from The Cancer Genome Atlas database showed that CAFs were associated with poor prognosis in solid-type cancer | (26-29,88-90) |
TIME, tumor immune microenvironment; APC, antigen-presenting cell; DCs, dendritic cells; MHC, major histocompatibility complex; MDSC, myeloid-derived suppressor cell; NSCLC, non-small cell lung cancer; PMN, polymorphonuclear leukocytes; HLA, human leucocyte antigen; NK, natural killer; CAF, cancer-associated fibroblast; VEGF, vascular endothelial growth factor; TAM, tumor-associated macrophage; IL, interleukin; TNF-α, tumor necrosis factor alpha; CCL, chemokine (C-C motif) ligand; PMN, polymorphonuclear leukocyte; α-SMA, alpha-smooth muscle actin; PDGF, platelet-derived growth factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; DFS, disease-free survival.
TIME heterogeneity
Overview of heterogeneity in the TIME
In management of advanced NSCLC, inhibiting cancer cell proliferation and if possible, killing them are the most important objectives. Therapeutic targeting of tumor cells becomes difficult, if cells within the same tumor exhibit various phenotypes, and all phenotypes simultaneously show different responses to anti-tumor treatment. Heterogeneity in TIME is important, because bigger the heterogeneity, more likely that tumor cells be irresponsive to anticancer treatment. Furthermore, when cancer progresses, more genetically and molecularly divergent lineages will come to exist, augmenting the TIME heterogeneity. For these reasons, it is important to understand the TIME heterogeneity and overcome it in order to increase treatment responses.
Several factors cause intra-tumoral heterogeneity including genetic and epigenetic alterations, extrinsic factors such as the tumor microenvironment, and interactions with other non-tumor cell types (e.g., CAFs and immune cells) (91). Heterogeneity in the TIME can be either spatial or temporal. Subsequent to heterogeneous changes in the TIME, treatment of lung cancer can be more challenging because drug resistance is more likely to occur.
Spatial heterogeneity in the TIME
The chemotactic factors secreted by the target organ and the intrinsic tendency of tumor cells to migrate to and proliferate at a specific site are important for metastatic potential (92). When tumor cells from primary lung cancer metastasize to distant sites, several steps are involved. Tumor cells travelling via the bloodstream escape immune surveillance and arrive at distant organs to form a metastatic niche. At the early phase of metastatic niche formation, infiltration by cancer cells involves degradation of the ECM. Cell-to-cell adhesions are weakened with increased levels of N-cadherin and integrin β1 while suppressing Serpin B2 (93,94). Tumor cells of metastatic lesions have a different tumor microenvironment from primary sites, and this heterogeneity can be a challenge in treatment of metastatic lung cancer.
Bone
Compared with other organs, bone is a relatively immunocompromised area and an environment in which cancer cells are more amenable to proliferation. In the pre-metastatic niche, there are large numbers of immature and inhibitory immune cells, a relatively smaller number of T cells, and a small proportion of NK cells in bone marrow (95,96). Conversely, Treg cells account for a large proportion of non-cytotoxic immune cells in bone, which co-exist with a large number of other inhibitory cells such as MDSCs (97).
Balance between osteoclasts and osteoblasts is an important feature in the tumor microenvironment. Cancer cells can induce imbalance between osteoblasts and osteoclasts and deter effective bone reconstruction (98). Lung cancer cells secrete IL-7, and T-cell-derived cytokines including TNF-α and receptor activator of nuclear factor-κB ligand (RANKL) are upregulated, further promoting osteoclast production (99). Osteolytic and osteogenic bone metastases can occur in lung cancer; osteolytic metastasis caused by osteoclasts is the predominant type (100). Osteoclasts can secrete various immunosuppression-inducing substances including indoleamine 2,3-dioxygenase-1 (IDO1) and IL-10. Bone resorption results in the release of TGF-β and IL-6 secretion, causing T cells to differentiate into T helper 17 and Treg cells, further contributing to formation of the TIME. The T helper 17 lymphocytes release IL-17 and IFN-γ, further promoting osteoclast differentiation (96).
Brain
The brain is one of the most frequently metastasized sites of NSCLC (101) and has several unique anatomic features. The blood-brain barrier (BBB) serves as protection against micrometastatic diseases; however, under certain circumstances, circulating tumor cells may cross the BBB via transendothelial migration (102,103). Subsequent to formation of the metastatic niche in the brain, cancer-associated angiogenesis, vascular remodeling, and changes in surface molecules occur (104). Despite the BBB, tumor-infiltrating T lymphocytes and other immune cells from systemic circulation can migrate to the brain metastatic lesions (105). Some subgroups of immune cells from the central nervous system (CNS) can also enter the endolymphatic system, into the cerebrospinal fluid, and travel to the lymphatic system. CD4-positive memory T cells and macrophages are present in the ventricle, pia mater, and other perivascular spaces and play important roles in immune monitoring in the brain (106).
Regarding management of brain metastatic disease in NSCLC, the disparity in tumor microenvironment between primary lung lesion and intracranial lesion should be considered. PD-L1 expression is an important biomarker predicting ICI efficacy in NSCLC. In a paired primary lung cancer and brain metastases study by Mansfield et al., the inconsistency rate of positive PD-L1 expression in paired cancer samples reached 14% despite some time interval between acquisition of the two samples (107). Compared with premetastatic lesions, patients with brain metastatic lung carcinoma show higher proportions of immunosuppressive peripheral monocyte PD-L1, MDSC, and Treg (108). Furthermore, brain metastatic lesions reportedly showed a larger fraction of tumor cells compared with primary lung tumors (109).
Liver
The hepatic metastatic site has a unique environment associated with local immune tolerance. The liver is a Kupffer cell-rich environment. Similar to other organs, metastatic cancer cells that reach the liver can trigger T-cell-mediated immune responses. Kupffer cells play various roles including cholesterol metabolism, pathogen removal, and initiation of local immunity (110). Mature Kupffer cells have a central role in immune surveillance by detecting, binding, and internalizing pathogens and other associated molecules such as lipopolysaccharide. Activated Kupffer cells release cytokines and chemokines to activate other immune cells (111). However, after invasion of cancer cells, Kupffer cells have both tumor-killing and pro-metastatic functions. In the early phase of metastasis, Kupffer cells kill and clear circulating metastatic cells. However, in the later phase, they can contribute to metastatic growth (112).
Despite various activities by immune cells in the tumor microenvironment, T cell-mediated anti-tumor activities can be deterred. Antigen presentation by local APC, usually a prerequisite for a cytotoxic T cell response, can also lead to immune tolerance. When expressing PD-1 ligands PD-L1 and PD-L2, the metastatic tumor cells can escape from CD4+ T helper cell- and CD8+ cytotoxic T lymphocyte (CTL)-mediated killing. Subsequent recruitment of MDSCs and Treg cells to the tumor microenvironment in the liver contributes to an immunosuppressive state. TGF-β and IL-2 further polarize naive T cells into inducible Treg cells, which further inhibit CD8+ T cell activity by releasing TGF-β, IL-10, granzymes, and perforin (81). The TGF-β-rich microenvironment induces neutrophils and monocytes to immunosuppressive (M2 and N2) phenotypes (113).
Similar to other metastatic organs, there are differences in the TIME between primary lung cancer and liver metastasis. In multiplexed IHC analysis of primary lung cancer and liver metastasis paired samples of 10 lung cancer patients, six immune markers (CD4, CD8, CTLA-4, granzyme B, FoxP3, and PD-L1) were evaluated. Primary lung cancer lesions showed higher number of tumor-infiltrating lymphocytes (TILs) and other T cells compared with liver metastasis. In addition, differences in CTLA-4 and PD-L1 expression were observed (114).
Changes in the TIME after anti-cancer treatment
When the tumor is exposed to systemic and local anti-cancer treatment and cancer cells survive, diverse subclones develop, temporal heterogeneity can occur (107). After patients undergo anti-cancer modalities such as chemotherapy, radiotherapy, and targeted therapies, PD-L1 expression show variable changes in tumor tissues, indicating the possibility of unpredictable immune-mediated cancer cell killing activities (115).
Reportedly, cytotoxic chemotherapy affects the TIME and, more specifically, the subpopulations of immune cells. Depletion of Treg cells, which play immunosuppressive roles in the TIME, can occur following cytotoxic chemotherapy regimens such as paclitaxel and cyclophosphamide (116,117). The analysis of peripheral blood samples of patients who underwent paclitaxel treatment showed that the inhibitory function of Treg was reduced, while the levels of interferon-γ (IFN-γ) and IL-2 were increased after paclitaxel treatment (116).
In a study including 138 epidermal growth factor receptor (EGFR) mutation-positive patients who underwent re-biopsy after progression while on EGFR-tyrosine kinase inhibitors (TKI) treatment, the treatment seemed to affect the TIME. The proportion of study patients with PD-L1 expression level of 50% or more significantly increased from 14% (before EGFR-TKI treatment) to 28% (after EGFR-TKI resistance). Densities of CD8+ and FoxP3+ TIL were significantly decreased after EGFR-TKI treatment (118).
In EGFR-mutated NSCLC, immunoinhibitory changes in the TIME reportedly occur in EGFR TKI-resistant NSCLC. Immunosuppressive cells were increased and immune-activated cells decreased in EGFR-TKI-resistant tumors compared with EGFR-TKI-sensitive tumors, and immune-inhibitory factors were more active in EGFR-TKI-resistant tumors. Furthermore, EGFR-TKI-resistant cancer cells exhibited epithelial–mesenchymal transition (119).
Radiotherapy induces different forms of changes in the TIME depending on radiation dose. Low-dose radiotherapy initiates the anti-tumor response, enabling NK and T cell infiltration into tumor cells by modulating the tumor stroma. However, there is minimal influence on cancer blood vessels or direct damage to the tumor. Conversely, high-dose radiotherapy is more likely to activate the strong anti-tumor immune response. Direct killing of tumor cells, subsequent antigen release, and T cell priming can occur. When used in combination, immunotherapy decreases T cell exhaustion and enhances lymphocyte activity against non-irradiated tumors. However, concurrent destruction results in large regions of hypoxia, which subsequently initiate processes leading to tumor regrowth (120,121).
Current strategies and future perspectives for TIME-targeting treatment
The TIME as a therapeutic target
In terms of the TIME, the main concept for increasing the chance of successful NSCLC treatment is to activate immune responses against tumor cells and inhibit immunosuppressive activities. In addition, relevant research is focused on normalizing an otherwise aberrant TIME in NSCLC patients. Potential therapeutic targets include immune cells, cytokine interactions, and non-immune cells such as fibroblasts or vessels.
Favorable TIME for immunotherapy
PD-L1 expression is the most widely known biomarker correlated with ICI response (122,123). However, based on real-life data, immunotherapy treatment can show outcomes inconsistent with PD-L1 expression, indicating that additional factors may affect immunotherapy mechanisms. Overall, high PD-L1 expression and TIL density in the tumor microenvironment are necessary conditions for favorable anti-PD-1 or anti-PD-L1 antibody efficacy (13,124).
In a retrospective cohort study of anti-PD-(L)1-treated NSCLC, the density of CD103+ CD8+ cells in tumor tissues showed significant association with improved progression-free survival (PFS) in patients receiving immunotherapy (75). In a retrospective study involving 39 NSCLC patients who received immunotherapy, tumoral CD8+ immune cell status was associated with the overall response (P<0.01). Conversely, 7 patients with high PD-L1 expression and low tumoral CD8+ did not show a significant response. Notably, all patients had EGFR mutations (125).
T effector (Teff) cells play a central role in cytotoxic cell death; however, Treg cells are associated with immune evasion of tumor cells. The Teff/Treg cell ratio showed potential prognostic and predictive values in many tumor types (126-128). Plasma cell signatures were also reported to have predictive value for improved OS in NSCLC patients receiving immunotherapy (129).
In general, active tumor killing T cell and low immunosuppressive cell signatures are favorable conditions for patients to receive immunotherapy. Table 5 shows a brief summary of current literature on favorable TIME for immunotherapy.
Table 5
| Component | Description |
|---|---|
| PD-L1 expression | High PD-L1 expression favorable for immunotherapy |
| TIL | High TIL density favorable for immunotherapy |
| CD 8 cell | Density of CD103+ CD8+ cells in tumor tissues showed significant association with improved progression-free survival in patients receiving immunotherapy |
| T effector cell | T effector cells play a central role in cytotoxic cell death; however, Treg cells are associated with immune evasion of tumor cells |
| T reg | Teff/Treg cell ratio showed potential prognostic and predictive values in many tumor types |
| Plasma cell | Plasma cell signatures were also reported to have predictive value for improved overall survival in non-small cell lung cancer patients receiving immunotherapy |
PD-L1, programmed death-ligand 1; TIL, tumor-infiltrating lymphocytes.
Radiation therapy as an immune enhancer
Radiotherapy is a treatment modality used for localized control of the tumor in management of NSCLC. Treatment mechanisms include direct killing of tumor cells via double-strand DNA damage induction and anti-tumor immune response modulation in both irradiated and non-irradiated tumors (130). Radiotherapy upregulates MHC class I expression, enabling the immune system to react to neoantigens released from tumor cells (131), and activates immunogenic cell death by inducing calreticulin expression on the tumor cell surface and releasing ATP and HMGB1 (132,133).
A difference in TIME between metastatic lesions and primary lung cancer has been suggested (109,134), and pre-emptive radiotherapy can be an effective tool in direct killing of tumor cells and activator of anti-tumor responses before subclone lineages of tumor cells become more complex.
A combinatorial approach using radiotherapy and ICIs in NSCLC patients with brain metastases resulted in a high intracranial local tumor control rate (135,136). In a retrospective study including 152 NSCLC patients with fewer than 4 metastatic lesions, the ICI plus radiotherapy group showed improved objective response rate (ORR) and PFS compared with the ICI only group. In addition, the out-of-field (abscopal effect) response rate reached 41.3% in the ICI plus radiotherapy group (137).
Abscopal effects are also important because radiotherapy tends to change the TIME to be more susceptible to ICIs. Irradiated tumor cells can play a role similar to that of a vaccine that activates the systemic adaptive immune response and promotes regression of a distant tumor (130). Often, ICIs are used in combination with radiotherapy, and non-irradiated sites may regress following radiation to a site of metastatic disease (138). The efficacy of ipilimumab and concurrent radiation treatment of a single metastatic site in 39 NSCLC patients who progressed on prior treatment was evaluated in a prospective study. The study showed the significant abscopal effect. Furthermore, increased serum interferon-β level after radiation and early dynamic changes in blood T cells were significant predictors of treatment response (139).
However, possibility of additional adverse events should be discussed at multidisciplinary team, because potential treatment-related toxicities of combinatorial management are much higher than the single-regimen therapy.
Overcoming immunosuppressive signals in the TIME
Inhibiting multiple immunosuppressive targets is another approach. A number of T cell inhibitory signals can be potential therapeutic targets. LAG3 is expressed by several immune cells such as CD4+ and CD8+ T cells and Tregs. In addition, LAG3 are an inhibitory regulator of T cell activity and related cytokine production (140,141). Combined blockade of LAG3 and PD-1 resulted in synergistic enhancement of CD8+ T cell cytotoxicity and reduced the number of Treg cells in the TIME (142).
Eftilagimod alpha, a soluble LAG-3 protein, plus pembrolizumab showed an overall response rate (ORR) of 33% in pembrolizumab-refractory melanoma patients and of 50% in PD-1-naïve melanoma patients (143). Furthermore, in PD-1/PD-L1 refractory metastatic NSCLC, therapy combining eftilagimod alpha and pembrolizumab was shown to have a favorable 6-month OS rate of 73% when used as second-line treatment in a phase II TACTI-002 trial (NCT03625323) (144).
Relatimab, a LAG-3-blocking antibody, was shown to improve PFS in melanomas when used in combination with nivolumab (145). NCT04623775 is a prospective randomized study in which the efficacy and safety of nivolumab plus relatlimab in combination with platinum doublet chemotherapy were compared with those of nivolumab plus platinum-doublet chemotherapy in patients with stage IV or recurrent NSCLC (146). This ongoing trial is expected to show new possibilities for overcoming the immunosuppressive component of TIME.
TGF-β is a cytokine involved in immunotherapy resistance (147). Bintrafusp alfa is comprised of the extracellular domain of the TGF-β receptor II fused to a human immunoglobulin G1 antibody blocking PD-L1. Bintrafusp alfa was assessed in the expansion cohort of NCT02517398, in which patients with advanced NSCLC were included. This phase I, open-label trial included 80 patients and showed a relatively favorable ORR efficacy of 17.5% and 25.0% for 500 mg and 1,200 mg doses, respectively (148). Galunisertib (LY2157299) is a selective TGF-β receptor type I kinase inhibitor. In an ongoing trial (NCT02423343), the efficacy and safety of orally administered galunisertib in combination with nivolumab were evaluated in refractory NSCLC and hepatocellular carcinoma (149).
Another strategy is to stimulate pro-immunogenic signals to enhance anti-tumor activities. CD40 is a member of the TNF receptor superfamily (150). APX005M, an agonistic antibody that binds to CD154, a ligand of CD40, activates the receptor and activates APCs, B cells, and monocytes. When administered in combination with other chemotherapy regimens, APX005M has shown promise in a metastatic pancreatic cancer phase Ib trial (151). NCT03123783, a phase I-II open-label study administering APX005M in combination with nivolumab to patients with NSCLC or metastatic melanoma is ongoing and expected to show results in the near future (152).
Management of angiogenesis
Maintaining constant migration of tumor-killing T cells into the TIME is important but not likely without a normal vascular supply. Tumor cells, especially when they are in hypoxic conditions, induce angiogenesis; however, leaky abnormal vessels are formed and contribute to further hypoxic and immunosuppressive conditions (153,154).
To induce more severe immunogenic conditions, it is important to normalize the vascular supply to the tumor microenvironment. Additional evidence indicates a clinically important relationship between angiogenesis and immune cell activities (155). In a 2-stage, phase II study, treatment outcomes were evaluated when adding bevacizumab to atezolizumab in metastatic NSCLC patients who showed disease progression after atezolizumab monotherapy. Enrolled patients received 1,200 mg atezolizumab every 3 weeks, and bevacizumab was combined with atezolizumab after radiographic progression was confirmed in the previous stage. The combination treatment showed a disease control rate of 87.5%, with a median PFS of 5.6 months (95% CI: 4.1–7.1) and OS of 14.0 months (156). In another phase II study, the lung-MAP nonmatch sub-study (S1800A), ramucirumab combined with pembrolizumab showed significant improvement in OS compared with investigators’ choice of treatment for patients with advanced NSCLC previously treated with ICI and chemotherapy (157). These two recent studies show the possibility of anti-angiogenic treatment combined with ICIs as a novel method for rendering the TIME more favorable to cancer management.
CAR-T therapy
The main concept for overcoming TIME heterogeneity is promoting immunogenicity-related cell death and subsequent T cell-dependent anti-tumor activity (158,159). The objective of chimeric antigen receptor (CAR)-T cell immunotherapy overlaps this concept. The emergence of CAR-T cell therapy provides a new approach for lung cancer management. Potential targets of CAR-T cell therapy in NSCLC include EGFR, human epidermal growth factor receptor 2 (HER2), mesothelin, mucin 1 (MUC1), prostate stem cell antigen (PSCA), tyrosine kinase-like orphan receptor 1 (ROR1), carcinoembryonic antigen, CD80/CD86, and PD-L1 (160), and many associated prospective trials are ongoing (161).
NCT03525782 is a trial that evaluates the safety of PD-1-knockout engineered anti-MUC1 CAR-T cells for treatment of stage IIIB–IV NSCLC. Among the 20 treated patients who received at least one cycle of anti-MUC1 CAR-T cell therapy, 11 showed stable disease and 9 had progressive disease. Common adverse events included systemic symptoms such as fever and chills (162). Several ongoing trials on efficacy of HER2-specific CAR-T cells (NCT03198052, NCT03500991, and NCT03696030) are expected to show results in the near future (163).
A combination of CAR-T cell therapy and immunotherapy has shown potential in overcoming tumor heterogeneity. In a phase I study, pembrolizumab combined with locally delivered, autologous, mesothelin-targeted CAR-T cell therapy, was effective and safe in malignant pleural diseases comprised of metastatic lung and breast cancers and malignant pleural mesothelioma. In 27 patients, the median OS was 23.9 months, with 2 patients showing complete metabolic response. In this phase I study, combination treatment was shown to induce polyclonal immunity to overcome heterogeneity in tumor antigens and a potential synergistic effect of combined regional CAR-T cells and PD-1 blockade. The study showed that combination of CAR T cells and pembrolizumab further expanded endogenous T-cell clones which can contribute to overcoming tumor antigen heterogeneity (164).
However, several challenges should be overcome for CAR-T therapy to become a mainstay treatment modality. First, lung cancer is a solid tumor. CAR-T cell therapy showed potential in the treatment of hematological malignancies. However, treatment of solid tumors using CAR-T showed relatively decreased efficacies due to poor trafficking, short-lasting effects, and limited T cell activities in the tumor microenvironment (165,166). Several T cell barriers including stromal tissue surrounding the tumor cells must be overcome. Second, lung cancer has a relatively small number of targetable antigens compared with other tumor types (167). Last, several potential target antigens are also expressed in normal tissues, imparting risk for CAR-T cells to attack normal cells (160).
Nanomedicine
Cancer nanomedicine has several advantages. Nanomedicine enables more accurate delivery to the target tissues. Due to its modular flexibility, anti-cancer medications can exist in various forms enabling more effective transportation and absorption. Nanomedicine has provided chances to promote anti-tumor immune responses and minimize unnecessary systemic side effects (168). Intratumor conditions can trigger anti-tumor actions of immunotherapy and may provide possible switch-on signals for localized tumor cell-killing effects. Tumor microenvironment-specific conditions regarding reactive oxygen species (ROS), pH, GSH, ATP, hypoxia, and cytokines are possible targets of cancer nanomedicine combined with immunotherapy (168). Acidic tumor environment is generally T cell inhibitory, and a pH-sensitive signaling pathway has recently been suggested as a candidate mechanism to balance localized activation of T cells in the tumor microenvironment while avoiding unwanted systemic immune responses (169).
Human studies on the efficacy of nanomedicine are limited and most were performed in vitro. Salvianolic acid B-loaded PEGylated liposomes were shown to induce inactivation of CAFs by inhibiting TGF-β1 secretion. Subsequently, collagen deposition in tumors was reduced and penetration of nanoparticles into tumors increased (170).
Conclusions
Immunosuppressive signals and heterogeneity in TIME are serious obstacles to treatment of NSCLC, and ICIs play essential roles. In management of lung cancer, understanding the heterogeneity and personalized analysis of lung cancer is significant for overcoming treatment resistance. Ongoing trials including combination therapy such as radiotherapy, cytotoxic chemotherapy, and anti-angiogenic treatment and regimens inhibiting other immunoinhibitory molecules are promising.
Acknowledgments
Funding: This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (No. 2019M3A9H2032425).
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-633/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-633/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-633/coif). SJK reports that this research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (No. 2019M3A9H2032425). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008;359:1367-80. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Paz-Ares L, Vicente D, Tafreshi A, et al. A Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients With Metastatic Squamous NSCLC: Protocol-Specified Final Analysis of KEYNOTE-407. J Thorac Oncol 2020;15:1657-69. [Crossref] [PubMed]
- Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N Engl J Med 2020;383:1328-39. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Fong L, Small EJ. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol 2008;26:5275-83. [Crossref] [PubMed]
- Wang S, Chen L. Immunobiology of cancer therapies targeting CD137 and B7-H1/PD-1 cosignal pathways. Curr Top Microbiol Immunol 2011;344:245-67. [Crossref] [PubMed]
- Bianco A, Perrotta F, Barra G, et al. Prognostic Factors and Biomarkers of Responses to Immune Checkpoint Inhibitors in Lung Cancer. Int J Mol Sci 2019;20:4931. [Crossref] [PubMed]
- Shinohara S, Takahashi Y, Komuro H, et al. New evaluation of the tumor immune microenvironment of non-small cell lung cancer and its association with prognosis. J Immunother Cancer 2022;10:e003765. [Crossref] [PubMed]
- Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci 2012;125:5591-6. [Crossref] [PubMed]
- Yu Y, Cui J. Present and future of cancer immunotherapy: A tumor microenvironmental perspective. Oncol Lett 2018;16:4105-13. [Crossref] [PubMed]
- Galli F, Aguilera JV, Palermo B, et al. Relevance of immune cell and tumor microenvironment imaging in the new era of immunotherapy. J Exp Clin Cancer Res 2020;39:89. [Crossref] [PubMed]
- Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 2018;24:541-50. [Crossref] [PubMed]
- Creaney J, Ma S, Sneddon SA, et al. Strong spontaneous tumor neoantigen responses induced by a natural human carcinogen. Oncoimmunology 2015;4:e1011492. [Crossref] [PubMed]
- Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002;3:991-8. [Crossref] [PubMed]
- Goc J, Germain C, Vo-Bourgais TK, et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res 2014;74:705-15. [Crossref] [PubMed]
- Cancel JC, Crozat K, Dalod M, et al. Are Conventional Type 1 Dendritic Cells Critical for Protective Antitumor Immunity and How? Front Immunol 2019;10:9. [Crossref] [PubMed]
- Binnewies M, Mujal AM, Pollack JL, et al. Unleashing Type-2 Dendritic Cells to Drive Protective Antitumor CD4(+) T Cell Immunity. Cell 2019;177:556-571.e16. [Crossref] [PubMed]
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39:1-10. [Crossref] [PubMed]
- Tang T, Huang X, Zhang G, et al. Advantages of targeting the tumor immune microenvironment over blocking immune checkpoint in cancer immunotherapy. Signal Transduct Target Ther 2021;6:72. [Crossref] [PubMed]
- Alroqi FJ, Chatila TA. T Regulatory Cell Biology in Health and Disease. Curr Allergy Asthma Rep 2016;16:27. [Crossref] [PubMed]
- Taams LS, Palmer DB, Akbar AN, et al. Regulatory T cells in human disease and their potential for therapeutic manipulation. Immunology 2006;118:1-9. [Crossref] [PubMed]
- Ohue Y, Nishikawa H, Regulatory T. Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci 2019;110:2080-9. [Crossref] [PubMed]
- Zhao S, Jiang T, Zhang L, et al. Clinicopathological and prognostic significance of regulatory T cells in patients with non-small cell lung cancer: A systematic review with meta-analysis. Oncotarget 2016;7:36065-73. [Crossref] [PubMed]
- Wang X, Xiao Z, Gong J, et al. A prognostic nomogram for lung adenocarcinoma based on immune-infiltrating Treg-related genes: from bench to bedside. Transl Lung Cancer Res 2021;10:167-82. [Crossref] [PubMed]
- Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009;324:1457-61. [Crossref] [PubMed]
- Öhlund D, Handly-Santana A, Biffi G, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 2017;214:579-96. [Crossref] [PubMed]
- Biffi G, Tuveson DA. Deciphering cancer fibroblasts. J Exp Med 2018;215:2967-8. [Crossref] [PubMed]
- Feig C, Jones JO, Kraman M, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A 2013;110:20212-7. [Crossref] [PubMed]
- Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer 2013;13:739-52. [Crossref] [PubMed]
- Gabrilovich DI. Myeloid-Derived Suppressor Cells. Cancer Immunol Res 2017;5:3-8. [Crossref] [PubMed]
- Elliott LA, Doherty GA, Sheahan K, et al. Human Tumor-Infiltrating Myeloid Cells: Phenotypic and Functional Diversity. Front Immunol 2017;8:86. [Crossref] [PubMed]
- Ortiz ML, Lu L, Ramachandran I, et al. Myeloid-derived suppressor cells in the development of lung cancer. Cancer Immunol Res 2014;2:50-8. [Crossref] [PubMed]
- Vatner RE, Formenti SC. Myeloid-derived cells in tumors: effects of radiation. Semin Radiat Oncol 2015;25:18-27. [Crossref] [PubMed]
- Xu F, Wei Y, Tang Z, et al. Tumor-associated macrophages in lung cancer: Friend or foe? Mol Med Rep 2020;22:4107-15. (Review). [PubMed]
- Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol 2014;192:5451-8. [Crossref] [PubMed]
- Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood 2006;107:2409-14. [Crossref] [PubMed]
- Emmerich J, Mumm JB, Chan IH, et al. IL-10 directly activates and expands tumor-resident CD8(+) T cells without de novo infiltration from secondary lymphoid organs. Cancer Res 2012;72:3570-81. [Crossref] [PubMed]
- Mirlekar B. Tumor promoting roles of IL-10, TGF-β, IL-4, and IL-35: Its implications in cancer immunotherapy. SAGE Open Med 2022;10:20503121211069012. [Crossref] [PubMed]
- Mu J, Zou JP, Yamamoto N, et al. Administration of recombinant interleukin 12 prevents outgrowth of tumor cells metastasizing spontaneously to lung and lymph nodes. Cancer Res 1995;55:4404-8. [PubMed]
- Snijders A, Kalinski P, Hilkens CM, et al. High-level IL-12 production by human dendritic cells requires two signals. Int Immunol 1998;10:1593-8. [Crossref] [PubMed]
- Bent EH, Millán-Barea LR, Zhuang I, et al. Microenvironmental IL-6 inhibits anti-cancer immune responses generated by cytotoxic chemotherapy. Nat Commun 2021;12:6218. [Crossref] [PubMed]
- Morishima N, Owaki T, Asakawa M, et al. Augmentation of effector CD8+ T cell generation with enhanced granzyme B expression by IL-27. J Immunol 2005;175:1686-93. [Crossref] [PubMed]
- Schneider R, Yaneva T, Beauseigle D, et al. IL-27 increases the proliferation and effector functions of human naïve CD8+ T lymphocytes and promotes their development into Tc1 cells. Eur J Immunol 2011;41:47-59. [Crossref] [PubMed]
- O'Connor RA, Chauhan V, Mathieson L, et al. T cells drive negative feedback mechanisms in cancer associated fibroblasts, promoting expression of co-inhibitory ligands, CD73 and IL-27 in non-small cell lung cancer. Oncoimmunology 2021;10:1940675. [Crossref] [PubMed]
- Pot C, Jin H, Awasthi A, et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol 2009;183:797-801. [Crossref] [PubMed]
- De Ridder K, Locy H, Piccioni E, et al. TNF-α-Secreting Lung Tumor-Infiltrated Monocytes Play a Pivotal Role During Anti-PD-L1 Immunotherapy. Front Immunol 2022;13:811867. [Crossref] [PubMed]
- Ardestani S, Li B, Deskins DL, et al. Membrane versus soluble isoforms of TNF-α exert opposing effects on tumor growth and survival of tumor-associated myeloid cells. Cancer Res 2013;73:3938-50. [Crossref] [PubMed]
- Vredevoogd DW, Kuilman T, Ligtenberg MA, et al. Augmenting Immunotherapy Impact by Lowering Tumor TNF Cytotoxicity Threshold. Cell 2020;180:404-5. [Crossref] [PubMed]
- Chen X, Bäumel M, Männel DN, et al. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J Immunol 2007;179:154-61. [Crossref] [PubMed]
- Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S, et al. B regulatory cells and the tumor-promoting actions of TNF-alpha during squamous carcinogenesis. Proc Natl Acad Sci U S A. 2011;108:10662-7. [Crossref] [PubMed]
- Fucikova J, Spisek R, Kroemer G, et al. Calreticulin and cancer. Cell Res 2021;31:5-16. [Crossref] [PubMed]
- Welch DR, Hurst DR. Defining the Hallmarks of Metastasis. Cancer Res 2019;79:3011-27. [Crossref] [PubMed]
- Hao Q, Tang H. Interferon-γ and Smac mimetics synergize to induce apoptosis of lung cancer cells in a TNFα-independent manner. Cancer Cell Int 2018;18:84. [Crossref] [PubMed]
- Liu C, Chikina M, Deshpande R, et al. Treg Cells Promote the SREBP1-Dependent Metabolic Fitness of Tumor-Promoting Macrophages via Repression of CD8+ T Cell-Derived Interferon-γ. Immunity 2019;51:381-97.e6. [Crossref] [PubMed]
- Huo JL, Wang YT, Fu WJ, et al. The promising immune checkpoint LAG-3 in cancer immunotherapy: from basic research to clinical application. Front Immunol 2022;13:956090. [Crossref] [PubMed]
- Zhang X, Zeng Y, Qu Q, et al. PD-L1 induced by IFN-γ from tumor-associated macrophages via the JAK/STAT3 and PI3K/AKT signaling pathways promoted progression of lung cancer. Int J Clin Oncol 2017;22:1026-33. [Crossref] [PubMed]
- Mezrich JD, Fechner JH, Zhang X, et al. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol 2010;185:3190-8. [Crossref] [PubMed]
- Huang X, Zhang F, Wang X, et al. The Role of Indoleamine 2, 3-Dioxygenase 1 in Regulating Tumor Microenvironment. Cancers (Basel) 2022;14:2756. [Crossref] [PubMed]
- Novitskiy SV, Ryzhov S, Zaynagetdinov R, et al. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood 2008;112:1822-31. [Crossref] [PubMed]
- Leone RD, Emens LA. Targeting adenosine for cancer immunotherapy. J Immunother Cancer 2018;6:57. [Crossref] [PubMed]
- Finetti F, Travelli C, Ercoli J, et al. Prostaglandin E2 and Cancer: Insight into Tumor Progression and Immunity. Biology (Basel) 2020;9:434. [Crossref] [PubMed]
- Luo F, Lu FT, Cao JX, et al. HIF-1α inhibition promotes the efficacy of immune checkpoint blockade in the treatment of non-small cell lung cancer. Cancer Lett 2022;531:39-56. [Crossref] [PubMed]
- Fu C, Jiang L, Hao S, et al. Activation of the IL-4/STAT6 Signaling Pathway Promotes Lung Cancer Progression by Increasing M2 Myeloid Cells. Front Immunol 2019;10:2638. [Crossref] [PubMed]
- Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity 2009;30:646-55. [Crossref] [PubMed]
- Tuminello S, Veluswamy R, Lieberman-Cribbin W, et al. Prognostic value of immune cells in the tumor microenvironment of early-stage lung cancer: a meta-analysis. Oncotarget 2019;10:7142-55. [Crossref] [PubMed]
- Kawai O, Ishii G, Kubota K, et al. Predominant infiltration of macrophages and CD8(+) T Cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer 2008;113:1387-95. [Crossref] [PubMed]
- Li L, Lu G, Liu Y, et al. Low Infiltration of CD8+ PD-L1+ T Cells and M2 Macrophages Predicts Improved Clinical Outcomes After Immune Checkpoint Inhibitor Therapy in Non-Small Cell Lung Carcinoma. Front Oncol 2021;11:658690. [Crossref] [PubMed]
- Stankovic B, Bjørhovde HAK, Skarshaug R, et al. Immune Cell Composition in Human Non-small Cell Lung Cancer. Front Immunol 2019;9:3101. [Crossref] [PubMed]
- Kurebayashi Y, Emoto K, Hayashi Y, et al. Comprehensive Immune Profiling of Lung Adenocarcinomas Reveals Four Immunosubtypes with Plasma Cell Subtype a Negative Indicator. Cancer Immunol Res 2016;4:234-47. [Crossref] [PubMed]
- Suzuki K, Kadota K, Sima CS, et al. Clinical impact of immune microenvironment in stage I lung adenocarcinoma: tumor interleukin-12 receptor beta2 (IL-12Rbeta2), IL-7R, and stromal FoxP3/CD3 ratio are independent predictors of recurrence. J Clin Oncol 2013;31:490-8. [Crossref] [PubMed]
- Leong TL, Bryant VL. B cells in lung cancer-not just a bystander cell: a literature review. Transl Lung Cancer Res 2021;10:2830-41. [Crossref] [PubMed]
- Germain C, Gnjatic S, Tamzalit F, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med 2014;189:832-44. [Crossref] [PubMed]
- Bruno TC, Ebner PJ, Moore BL, et al. Antigen-Presenting Intratumoral B Cells Affect CD4(+) TIL Phenotypes in Non-Small Cell Lung Cancer Patients. Cancer Immunol Res 2017;5:898-907. [Crossref] [PubMed]
- Corgnac S, Malenica I, Mezquita L, et al. CD103(+)CD8(+) T(RM) Cells Accumulate in Tumors of Anti-PD-1-Responder Lung Cancer Patients and Are Tumor-Reactive Lymphocytes Enriched with Tc17. Cell Rep Med 2020;1:100127. [Crossref] [PubMed]
- Gebhardt T, Palendira U, Tscharke DC, et al. Tissue-resident memory T cells in tissue homeostasis, persistent infection, and cancer surveillance. Immunol Rev 2018;283:54-76. [Crossref] [PubMed]
- Byrne A, Savas P, Sant S, et al. Tissue-resident memory T cells in breast cancer control and immunotherapy responses. Nat Rev Clin Oncol 2020;17:341-8. [Crossref] [PubMed]
- Dai F, Liu L, Che G, et al. The number and microlocalization of tumor-associated immune cells are associated with patient's survival time in non-small cell lung cancer. BMC Cancer 2010;10:220. [Crossref] [PubMed]
- Yamauchi Y, Safi S, Blattner C, et al. Circulating and Tumor Myeloid-derived Suppressor Cells in Resectable Non-Small Cell Lung Cancer. Am J Respir Crit Care Med 2018;198:777-87. [Crossref] [PubMed]
- Wang PF, Song SY, Wang TJ, et al. Prognostic role of pretreatment circulating MDSCs in patients with solid malignancies: A meta-analysis of 40 studies. Oncoimmunology 2018;7:e1494113. [Crossref] [PubMed]
- Ciner AT, Jones K, Muschel RJ, et al. The unique immune microenvironment of liver metastases: Challenges and opportunities. Semin Cancer Biol 2021;71:143-56. [Crossref] [PubMed]
- Duan J, Lv G, Zhu N, et al. Multidimensional profiling depicts infiltrating immune cell heterogeneity in the tumor microenvironment of stage IA non-small cell lung cancer. Thorac Cancer 2022;13:947-55. [Crossref] [PubMed]
- Yoshida C, Kadota K, Yamada K, et al. Tumor-associated CD163(+) macrophage as a predictor of tumor spread through air spaces and with CD25(+) lymphocyte as a prognostic factor in resected stage I lung adenocarcinoma. Lung Cancer 2022;167:34-40. [Crossref] [PubMed]
- Vivier E, Artis D, Colonna M, et al. Innate Lymphoid Cells: 10 Years On. Cell 2018;174:1054-66. [Crossref] [PubMed]
- Villegas FR, Coca S, Villarrubia VG, et al. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer 2002;35:23-8. [Crossref] [PubMed]
- Takanami I, Takeuchi K, Giga M. The prognostic value of natural killer cell infiltration in resected pulmonary adenocarcinoma. J Thorac Cardiovasc Surg 2001;121:1058-63. [Crossref] [PubMed]
- Platonova S, Cherfils-Vicini J, Damotte D, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res 2011;71:5412-22. [Crossref] [PubMed]
- Roulis M, Flavell RA. Fibroblasts and myofibroblasts of the intestinal lamina propria in physiology and disease. Differentiation 2016;92:116-31. [Crossref] [PubMed]
- Ishii G, Ochiai A, Neri S. Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumor microenvironment. Adv Drug Deliv Rev 2016;99:186-96. [Crossref] [PubMed]
- Min KW, Kim DH, Noh YK, et al. Cancer-associated fibroblasts are associated with poor prognosis in solid type of lung adenocarcinoma in a machine learning analysis. Sci Rep 2021;11:16779. [Crossref] [PubMed]
- Sun XX, Yu Q. Intra-tumor heterogeneity of cancer cells and its implications for cancer treatment. Acta Pharmacol Sin 2015;36:1219-27. [Crossref] [PubMed]
- Wood SL, Pernemalm M, Crosbie PA, et al. The role of the tumor-microenvironment in lung cancer-metastasis and its relationship to potential therapeutic targets. Cancer Treat Rev 2014;40:558-66. [Crossref] [PubMed]
- Bae SY, Park HJ, Hong JY, et al. Down-regulation of SerpinB2 is associated with gefitinib resistance in non-small cell lung cancer and enhances invadopodia-like structure protrusions. Sci Rep 2016;6:32258. [Crossref] [PubMed]
- Yamauchi M, Yoshino I, Yamaguchi R, et al. N-cadherin expression is a potential survival mechanism of gefitinib-resistant lung cancer cells. Am J Cancer Res 2011;1:823-33. [PubMed]
- Zhao E, Xu H, Wang L, et al. Bone marrow and the control of immunity. Cell Mol Immunol 2012;9:11-9. [Crossref] [PubMed]
- Del Conte A, De Carlo E, Bertoli E, et al. Bone Metastasis and Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer (NSCLC): Microenvironment and Possible Clinical Implications. Int J Mol Sci 2022;23:6832. [Crossref] [PubMed]
- Zou L, Barnett B, Safah H, et al. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res 2004;64:8451-5. [Crossref] [PubMed]
- Xu S, Yang F, Liu R, et al. Serum microRNA-139-5p is downregulated in lung cancer patients with lytic bone metastasis. Oncol Rep 2018;39:2376-84. [Crossref] [PubMed]
- Roato I, Caldo D, Godio L, et al. Bone invading NSCLC cells produce IL-7: mice model and human histologic data. BMC Cancer 2010;10:12. [Crossref] [PubMed]
- Wu S, Pan Y, Mao Y, et al. Current progress and mechanisms of bone metastasis in lung cancer: a narrative review. Transl Lung Cancer Res 2021;10:439-51. [Crossref] [PubMed]
- Riihimäki M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung Cancer 2014;86:78-84. [Crossref] [PubMed]
- Banks WA. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov 2016;15:275-92. [Crossref] [PubMed]
- Sprowls SA, Arsiwala TA, Bumgarner JR, et al. Improving CNS Delivery to Brain Metastases by Blood-Tumor Barrier Disruption. Trends Cancer 2019;5:495-505. [Crossref] [PubMed]
- Lockman PR, Mittapalli RK, Taskar KS, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res 2010;16:5664-78. [Crossref] [PubMed]
- Berghoff AS, Fuchs E, Ricken G, et al. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunology 2015;5:e1057388. [Crossref] [PubMed]
- Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol 2012;12:623-35. [Crossref] [PubMed]
- Mansfield AS, Aubry MC, Moser JC, et al. Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann Oncol 2016;27:1953-8. [Crossref] [PubMed]
- Li YD, Lamano JB, Lamano JB, et al. Tumor-induced peripheral immunosuppression promotes brain metastasis in patients with non-small cell lung cancer. Cancer Immunol Immunother 2019;68:1501-13. [Crossref] [PubMed]
- Paik PK, Shen R, Won H, et al. Next-Generation Sequencing of Stage IV Squamous Cell Lung Cancers Reveals an Association of PI3K Aberrations and Evidence of Clonal Heterogeneity in Patients with Brain Metastases. Cancer Discov 2015;5:610-21. [Crossref] [PubMed]
- Keirsse J, Van Damme H, Geeraerts X, et al. The role of hepatic macrophages in liver metastasis. Cell Immunol 2018;330:202-15. [Crossref] [PubMed]
- Kubes P, Jenne C. Immune Responses in the Liver. Annu Rev Immunol 2018;36:247-77. [Crossref] [PubMed]
- Wen SW, Ager EI, Christophi C. Bimodal role of Kupffer cells during colorectal cancer liver metastasis. Cancer Biol Ther 2013;14:606-13. [Crossref] [PubMed]
- Chanmee T, Ontong P, Konno K, et al. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6:1670-90. [Crossref] [PubMed]
- Cho H, Kim JH, Kim JH. 747 Evaluation of immune microenvironment of primary lung cancer and synchronous liver metastasis with multispectral imaging system. Journal for ImmunoTherapy of Cancer 2020;8:A448-9.
- Hong L, Negrao MV, Dibaj SS, et al. Programmed Death-Ligand 1 Heterogeneity and Its Impact on Benefit From Immune Checkpoint Inhibitors in NSCLC. J Thorac Oncol 2020;15:1449-59. [Crossref] [PubMed]
- Zhang L, Dermawan K, Jin M, et al. Differential impairment of regulatory T cells rather than effector T cells by paclitaxel-based chemotherapy. Clin Immunol 2008;129:219-29. [Crossref] [PubMed]
- Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol 2004;34:336-44. [Crossref] [PubMed]
- Isomoto K, Haratani K, Hayashi H, et al. Impact of EGFR-TKI Treatment on the Tumor Immune Microenvironment in EGFR Mutation-Positive Non-Small Cell Lung Cancer. Clin Cancer Res 2020;26:2037-46. [Crossref] [PubMed]
- Liu L, Wang C, Li S, et al. Tumor immune microenvironment in epidermal growth factor receptor-mutated non-small cell lung cancer before and after epidermal growth factor receptor tyrosine kinase inhibitor treatment: a narrative review. Transl Lung Cancer Res 2021;10:3823-39. [Crossref] [PubMed]
- Jarosz-Biej M, Smolarczyk R, Cichoń T, et al. Tumor Microenvironment as A "Game Changer" in Cancer Radiotherapy. Int J Mol Sci 2019;20:3212. [Crossref] [PubMed]
- Menon H, Chen D, Ramapriyan R, et al. Influence of low-dose radiation on abscopal responses in patients receiving high-dose radiation and immunotherapy. J Immunother Cancer 2019;7:237. [Crossref] [PubMed]
- Antonia SJ. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2019;380:990. Reply. [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Son B, Lee S, Youn H, et al. The role of tumor microenvironment in therapeutic resistance. Oncotarget 2017;8:3933-45. [Crossref] [PubMed]
- Shimoda Y, Shibaki R, Yoshida T, et al. Concurrent High PD-L1 Expression and CD8(+) Immune Cell Infiltration Predict PD-1 Blockade Efficacy in Advanced EGFR-Mutant NSCLC Patients. Clin Lung Cancer 2022;23:477-86. [Crossref] [PubMed]
- Murciano-Goroff YR, Warner AB, Wolchok JD. The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res 2020;30:507-19. [Crossref] [PubMed]
- Baras AS, Drake C, Liu JJ, et al. The ratio of CD8 to Treg tumor-infiltrating lymphocytes is associated with response to cisplatin-based neoadjuvant chemotherapy in patients with muscle invasive urothelial carcinoma of the bladder. Oncoimmunology 2016;5:e1134412. [Crossref] [PubMed]
- Preston CC, Maurer MJ, Oberg AL, et al. The ratios of CD8+ T cells to CD4+CD25+ FOXP3+ and FOXP3- T cells correlate with poor clinical outcome in human serous ovarian cancer. PLoS One 2013;8:e80063. [Crossref] [PubMed]
- Teillaud JL, Dieu-Nosjean MC. Intratumoral plasma cells: More than a predictive marker of response to anti-PD-L1 treatment in lung cancer? Cancer Cell 2022;40:240-3. [Crossref] [PubMed]
- Meng L, Xu J, Ye Y, et al. The Combination of Radiotherapy With Immunotherapy and Potential Predictive Biomarkers for Treatment of Non-Small Cell Lung Cancer Patients. Front Immunol 2021;12:723609. [Crossref] [PubMed]
- Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006;203:1259-71. [Crossref] [PubMed]
- Golden EB, Frances D, Pellicciotta I, et al. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology 2014;3:e28518. [Crossref] [PubMed]
- Li G, Liang X, Lotze MT. HMGB1: The Central Cytokine for All Lymphoid Cells. Front Immunol 2013;4:68. [Crossref] [PubMed]
- Tsakonas G, Lewensohn R, Botling J, et al. An immune gene expression signature distinguishes central nervous system metastases from primary tumours in non-small-cell lung cancer. Eur J Cancer 2020;132:24-34. [Crossref] [PubMed]
- Kim DY, Kim PH, Suh CH, et al. Immune Checkpoint Inhibitors with or without Radiotherapy in Non-Small Cell Lung Cancer Patients with Brain Metastases: A Systematic Review and Meta-Analysis. Diagnostics (Basel) 2020;10:1098. [Crossref] [PubMed]
- Suwinski R. Combination of immunotherapy and radiotherapy in the treatment of brain metastases from non-small cell lung cancer. J Thorac Dis 2021;13:3315-22. [Crossref] [PubMed]
- Wang P, Yin T, Zhao K, et al. Efficacy of single-site radiotherapy plus PD-1 inhibitors vs PD-1 inhibitors for oligometastatic non-small cell lung cancer. J Cancer Res Clin Oncol 2022;148:1253-61. [Crossref] [PubMed]
- Ngwa W, Irabor OC, Schoenfeld JD, et al. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer 2018;18:313-22. [Crossref] [PubMed]
- Formenti SC, Rudqvist NP, Golden E, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med 2018;24:1845-51. [Crossref] [PubMed]
- Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016;44:989-1004. [Crossref] [PubMed]
- Qin S, Xu L, Yi M, et al. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer 2019;18:155. [Crossref] [PubMed]
- Huang RY, Eppolito C, Lele S, et al. LAG3 and PD1 co-inhibitory molecules collaborate to limit CD8+ T cell signaling and dampen antitumor immunity in a murine ovarian cancer model. Oncotarget 2015;6:27359-77. [Crossref] [PubMed]
- Atkinson V, Khattak A, Haydon A, et al. Eftilagimod alpha, a soluble lymphocyte activation gene-3 (LAG-3) protein plus pembrolizumab in patients with metastatic melanoma. J Immunother Cancer 2020;8:e001681. [Crossref] [PubMed]
- OncLive. Second-Line Eftilagimod Alpha Plus Pembrolizumab Shows Promising Activity in Metastatic Lung Cancer. 2022.
- Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N Engl J Med 2022;386:24-34. [Crossref] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04623775. Accessed on Aug 2, 2022.
- Pickup M, Novitskiy S, Moses HL. The roles of TGFβ in the tumour microenvironment. Nat Rev Cancer 2013;13:788-99. [Crossref] [PubMed]
- Paz-Ares L, Kim TM, Vicente D, et al. Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGF-β and PD-L1, in Second-Line Treatment of Patients With NSCLC: Results From an Expansion Cohort of a Phase 1 Trial. J Thorac Oncol 2020;15:1210-22. [Crossref] [PubMed]
- NCT02423343 [Internet]. Accessed on Aug 13, 2022.
- Vonderheide RH. CD40 Agonist Antibodies in Cancer Immunotherapy. Annu Rev Med 2020;71:47-58. [Crossref] [PubMed]
- O'Hara MH, O'Reilly EM, Varadhachary G, et al. CD40 agonistic monoclonal antibody APX005M (sotigalimab) and chemotherapy, with or without nivolumab, for the treatment of metastatic pancreatic adenocarcinoma: an open-label, multicentre, phase 1b study. Lancet Oncol 2021;22:118-31. [Crossref] [PubMed]
- NCT03123783 [Internet]. Accessed on Aug 13, 2022.
- Abou Khouzam R, Brodaczewska K, Filipiak A, et al. Tumor Hypoxia Regulates Immune Escape/Invasion: Influence on Angiogenesis and Potential Impact of Hypoxic Biomarkers on Cancer Therapies. Front Immunol 2021;11:613114. [Crossref] [PubMed]
- Petrova V, Annicchiarico-Petruzzelli M, Melino G, et al. The hypoxic tumour microenvironment. Oncogenesis 2018;7:10. [Crossref] [PubMed]
- Manegold C, Dingemans AC, Gray JE, et al. The Potential of Combined Immunotherapy and Antiangiogenesis for the Synergistic Treatment of Advanced NSCLC. J Thorac Oncol 2017;12:194-207. [Crossref] [PubMed]
- Lee J, Koh J, Kim HK, et al. Bevacizumab Plus Atezolizumab After Progression on Atezolizumab Monotherapy in Pretreated Patients With NSCLC: An Open-Label, Two-Stage, Phase 2 Trial. J Thorac Oncol 2022;17:900-8. [Crossref] [PubMed]
- Reckamp KL, Redman MW, Dragnev KH, et al. Phase II Randomized Study of Ramucirumab and Pembrolizumab Versus Standard of Care in Advanced Non-Small-Cell Lung Cancer Previously Treated With Immunotherapy-Lung-MAP S1800A. J Clin Oncol 2022;40:2295-306. [Crossref] [PubMed]
- Gupta A, Probst HC, Vuong V, et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol 2012;189:558-66. [Crossref] [PubMed]
- Vincent J, Mignot G, Chalmin F, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res 2010;70:3052-61. [Crossref] [PubMed]
- Zhong S, Cui Y, Liu Q, et al. CAR-T cell therapy for lung cancer: a promising but challenging future. J Thorac Dis 2020;12:4516-21. [Crossref] [PubMed]
- Chen L, Chen F, Li J, et al. CAR-T cell therapy for lung cancer: Potential and perspective. Thorac Cancer 2022;13:889-99. [Crossref] [PubMed]
- Lin Y, Chen S, Zhong S, et al. 35O - Phase I clinical trial of PD-1 knockout anti-MUC1 CAR-T cells in the treatment of patients with non-small cell lung cancer. Ann Oncol 2019;30:xi12.
- Zeng J, Ma W, Young RB, et al. Targeting HER2 genomic alterations in non-small cell lung cancer. Journal of the National Cancer Center 2021;1:58-73. [Crossref]
- Adusumilli PS, Zauderer MG, Rivière I, et al. A Phase I Trial of Regional Mesothelin-Targeted CAR T-cell Therapy in Patients with Malignant Pleural Disease, in Combination with the Anti-PD-1 Agent Pembrolizumab. Cancer Discov 2021;11:2748-63. [Crossref] [PubMed]
- Yeku OO, Purdon TJ, Koneru M, et al. Armored CAR T cells enhance antitumor efficacy and overcome the tumor microenvironment. Sci Rep 2017;7:10541. [Crossref] [PubMed]
- Ye B, Stary CM, Li X, et al. Engineering chimeric antigen receptor-T cells for cancer treatment. Mol Cancer 2018;17:32. [Crossref] [PubMed]
- Zeltsman M, Dozier J, McGee E, et al. CAR T-cell therapy for lung cancer and malignant pleural mesothelioma. Transl Res 2017;187:1-10. [Crossref] [PubMed]
- Peng S, Xiao F, Chen M, et al. Tumor-Microenvironment-Responsive Nanomedicine for Enhanced Cancer Immunotherapy. Adv Sci (Weinh) 2022;9:e2103836. [Crossref] [PubMed]
- Alfei F, Ho PC, Lo WL. DCision-making in tumors governs T cell anti-tumor immunity. Oncogene 2021;40:5253-61. [Crossref] [PubMed]
- Chen Y, Hu M, Wang S, et al. Nano-delivery of salvianolic acid B induces the quiescence of tumor-associated fibroblasts via interfering with TGF-β1/Smad signaling to facilitate chemo- and immunotherapy in desmoplastic tumor. Int J Pharm 2022;623:121953. [Crossref] [PubMed]


