
Molecular cross-talk between the liver and white adipose tissue links excessive noURIshment to hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide and the second leading cause of cancer-related deaths (1). Traditionally, HCC develops in the presence of hepatic inflammation and damage due to the presence of hepatitis B viruses or hepatitis C viruses (HBV or HCV), or excessive alcohol consumption. However, recent trends and experimental data have shown that a major risk factor for HCC is obesity, which is associated with a high caloric intake and a sedentary lifestyle (2). Obesity increases hepatocellular lipid accumulation (steatohepatitis) and consequently causes non-alcoholic fatty acid liver disease (NAFLD), which when combined with chronic liver inflammation or injury, can develop into non-alcoholic steatohepatitis (NASH) and HCC. However, the molecular mechanisms linking obesity with NASH and HCC are not well understood, which hinders the development of treatment options. A major step forward for HCC research has been the discovery of the oncogene unconventional prefoldin RPB5 interactor (URI) and its requirement in cancer progression (3,4). URI is upregulated in tumors from HCC patients, as well as in HCC cell lines (5). In other cancers, higher URI genomic amplification, which occurs in 10% of ovarian cancers (6) and 40% of uterine carcinosarcoma (7), results in poorer prognosis for patients.
URI is a component of the R2TP/URI-prefoldin like complex that coordinates nutrient signaling by interacting with various proteins including heat shock protein 90 (HSP90) and transcriptional repressor RNA polymerase subunit RPB5 (8). Its function is regulated by the mTOR pathway, with S6 kinase 1 (S6K1) being directly responsible for URI phosphophorylation and activation, after stimulation from growth factors (9). Phosphorylation of URI releases the URI-bound protein phosphatase 1 (PP1γ) and decreases phosphorylation of the pro-apoptotic molecule BAD. As a result, the overexpression of URI can promote the progression of HCC by inhibiting apoptosis of cancer cells (5,9). Interestingly, the ability of URI to inhibit PP1γ did not have an effect in HCC mice models with overexpressed human URI (hURI) (4). In these models, URI affected the catabolism of tryptophan, by inhibiting the aryl hydrocarbon receptors (AhR) and estrogen receptors (ER), resulting in reduced nicotinamide adenine dinucleotide (NAD+) levels and initiating HCC by DNA damage, followed by hepatic inflammation (4).
Following on from this work, Gomes and colleagues recently reported a novel, essential role for URI in developing steatohepatitis and liver injury in response to mice fed NASH-inducing diets (10). URI expression levels were higher in C57BL/6 mice fed a choline-deficient high fat diet (CD-HFD) to induce NASH symptoms. Heterozygous deletion of URI [URI(+/Δ)hep] in high fat diet (HFD) fed mice resulted in lower hepatic lipid accumulation than wild type. A previously developed knock in model of HCC, with hepatic expression of hURI (hURI-tetOFFhep) (4), was used to confirm the role of URI in NASH. The development of steatohepatitis, fibrosis and liver injury was induced in hURI-tetOFFhep mutants, but reduced when hURI expression was blocked after 8 weeks with doxycycline. These results indicate that the development of steatohepatitis and chronic liver injury requires the continual expression of URI.
The expression of hURI increases the hepatic infiltration of T-cells and macrophages, likely Kupffer cells, and again this inflammation decreases with the blocking of hURI expression. As with the previous study (4), a HFD and/or hURI expression was shown to induce DNA damage, which preceded inflammation. A role for URI in DNA damage was first postulated by its association with PDRG1 (p53 and DNA damage regulated gene) (11), and confirmed by observing that URI induces the phosphorylation of p53 and the DNA damage response marker histone H2AX (γH2AX) (4). In this light, Gomes and colleagues showed that chemical inhibition of DNA damage through in vivo treatment with nicotinamide riboside (NR) was able to abrogate URI-induced inflammation in hURI expression mice (10). Notably, NR treatment also reduced DNA damage and inflammation in HFD-fed mice. Therefore, this study showed, for the first time, that URI expression is able to mimic the genotoxic stress induced by nutrient excess, mechanistically linking obesity to HCC development.
Interestingly, hepatic URI expression is also essential for neutrophil infiltration in white adipose tissue (WAT), as blocking hURI abolished recruitment. This intra-organ signaling between the liver and WAT is an important mechanism driving hepatosteatosis. The authors showed that increased hepatosteatosis was due to increased free fatty acid (FFA) uptake as opposed to de novo lipid synthesis, as fatty acid biosynthetic enzymes were downregulated in hURI-tetOFFhep mutants. In contrast, lipid uptake and fatty acid transporters were upregulated compared to wild type and URI(+/Δ)hep mice. As hURI-tetOFFhep expression mice had lower body mass than WT, which could be restored by blocking hURI, the authors suggested that URI-induced hepatosteatosis resulted from WAT lipolysis. This hypothesis was supported by the induction of insulin resistance (IR) and glucose intolerance in the presence of high URI levels. Under conditions of high URI expression, as in hURI-tetOFFhep mutants, there was an inability to regulate glucose and lipid levels, with increased serum levels of insulin, leptin and resistin, and decreased serum adiponectin. Furthermore, URI-induced WAT IR increases lipolysis and consequently NASH in mice, as hURI expression resulted in downregulation of insulin receptor substrate 1 and increased activation of lipolytic hormone-sensitive ligase. These results also have important implications for the development of type 2 diabetes (T2D), as hURI overexpression also induced a pre-diabetic state, with chronic systemic inflammation, intra-islet inflammation and vascular dilation observed in the pancreas of mutants. Therefore, in the context of elevated hepatic URI it is likely that the presence of IR and T2D are risk factors for NASH and HCC.
The systemic inflammation observed in HFD mice and hURI expression mutants is caused by chronically high levels of interleukin-17A (IL-17A) found in the blood, liver and WAT. IL-17A is a cytokine important for inducing neutrophil infiltration, and has been implicated in HCC tumorigenesis (12,13). Gomes et al. observed a link between URI and IL-17A expression, with reduced levels of IL-17A detected in URI(+/Δ)hep heterozygous knock out mice, and higher levels of the cytokine in hURI overexpression mutants (10). High IL-17A levels were also accompanied by increased levels of the IL-17A targets, granulocyte-colony stimulating factor (GCSF) and lipocalin 2. Notably, the homozygous knock out of the IL-17A receptor (IL17-RA) in myeloid cells abolished leukocytosis in HFD-fed mice, indicating the importance of IL-17A in the onset of obesity-triggered inflammation. A positive effect of IL-17A on hepatic URI levels was also detected, with increased URI expression when chow fed mice were injected with recombinant IL-17A, which consequently induced the development of WAT IR and lipolysis and in the liver, NASH, inflammation and fibrosis. Thus, the authors discovered a novel feed-forward loop between URI and IL-17A that positively regulates both proteins to trigger steatohepatitis and HCC development (Figure 1).
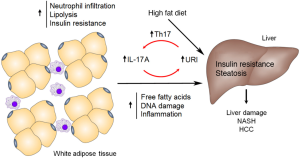
The role of IL-17A in NASH and HCC was further demonstrated through the observation of reduced levels of liver and circulating 1L-17A-expressing T helper 17 (Th17) cells in heterozygous URI knock out mice. Additionally, low URI levels decreased the number of cells expressing RAR-related orphan receptor γt (RORγt), the transcription factor responsible for Th17 differentiation. Therefore, hepatic URI expression is an important factor for mediating Th17-induced systemic inflammation. Chemical suppression of Th17 cell differentiation via digoxin decreased the circulating Th17 cell levels and the concentration of serum and WAT IL-17A. As a result, chemical inhibition of Th17 cells overcame the effects of hURI overexpression in mice, linking URI to Th17 recruitment and IL-17A induction. Specifically, digoxin reduced hepatosteatosis, hepatic inflammation, liver injury, FFA levels, IR, glucose intolerance, and even HCC tumors, with corresponding reductions in WAT lipolysis and IL-17A levels. Inhibition of Th17 differentiation also reduced the severity of tumors, steatosis, neutrophil infiltration and liver injury when a HFD and diethylnitrosamine (DEN) was used to induce HCC in mice. Similarly, blocking IL-17A with specific antibodies reduced hepatosteatosis, fibrosis and tumorigenesis, with a reduction in FFA levels, immune cell infiltration, and WAT IR and lipolysis. It is likely that the induction of Th17 cell differentiation is mediated by URI through an increase in interleukin 6 (IL-6) levels by inducing the transcriptional efficiency of nuclear factor-κB (NFκB) p65. In vitro knock down of URI in multiple myeloma cells was shown to decrease the expression of IL-6 and phosphorylation of NFκB p65 and oncogenic signal transducer and activator of transcription 3 (STAT3) (14). IL-17 and IL-6 are involved in another feed-forward loop, where IL-17 induces production of hepatocyte-stimulating factor IL-6 to activate the STAT3 pathway in HCC (12), and increased IL-6 promotes differentiation of Th17 cells to increase IL-17 secretion via STAT3 (15). In the study by Gomes et al., STAT3 levels were lower in HFD-fed URI(+/Δ)hep mice and higher in hURI-tetOFFhep mutants, while hepatic URI expression affected p65 levels in some liver inflammatory cells (10). However, levels of IL-6 expression and STAT-3 phosphorylation were not reported. Ostensibly, URI is involved in the activation of both IL-17 and IL-6 expression to initiate NASH and HCC.
Further convincing evidence of the involvement of IL-17A in liver cancer included analysis of human HCC samples. These analyses indicated high levels of IL-17A and IL-17A receptor (IL-17RA) in HCC samples, which correlated positively with URI expression. Interestingly, high URI expression in human liver tumors was associated with increased IL-17A levels in peritumoral tissues, suggesting a paracrine signaling role for this cytokine in inflammation and HCC development. In order to test this hypothesis, the inhibition of IL-17A signaling was achieved by the use of a homozygous knock out of the IL-17RA in myeloid cells. Significant reductions in hepatosteatosis, liver and WAT inflammation, FFA levels and IR were observed for IL-17RA mice. Critically, genetic crosses of the IL-17RA mutant with the hURI-tetOFFhep mice ablated hepatosteatosis, NASH and HCC formation, despite the sustained expression of hURI. Therefore, targeting IL-17A or IL-17A signaling may be an effective strategy for treating obesity-linked HCC, particularly as anti-IL-17A drugs have recently been approved for the treatment of other inflammatory diseases (16). However, further studies are required to determine the efficacy of long term treatment with IL-17A inhibitors for use in HCC treatment, particularly as the mTORC1 inhibitor rapamycin has been shown to reduce hepatosteatosis but increase tumorigenesis (17), and trials in humans have failed (18). Additionally, caution is necessary as IL-17 seems to play a complex Janus-faced role in tumorigenesis, with various anti- and pro-tumor activities depending on tissue type and host immunity (15).
The clinical relevance of URI and IL-17A in obesity-linked hepatosteatosis was confirmed by detecting high URI levels in livers from obese patients, but not in peritumoral samples from non-obese individuals (10). The URI levels also correlated with IL-17A protein and mRNA levels in these patients. Additionally, steatosis and hepatic triglyceride levels paralleled positively to the number of IL-17A+ cells and to IL-17A mRNA levels, respectively. Apart from NASH-HCC, interestingly and convincingly, URI was detected in all hepatitis C positive (HCV+) NASH patients, but only in half of the non-steatotic HCV+ individuals. Again, the presence of IL-17A+ cells correlated highly with the detection of URI, and these cells were only observed in steatotic patients. This feed-forward molecular loop is likely to be unique to NASH-driven HCC, as a positive association between URI and IL-17A levels was observed in HCC samples from hepatitis-infected patients, but not in HCC samples from patients with alcoholic steatohepatitis.
This study presents an important breakthrough in determining the molecular mechanisms driving obesity-triggered HCC. The authors present a compelling argument to demonstrate that URI and IL-17A are involved in a feed-forward loop that drives DNA damage, inflammation and hepatosteatosis, leading to NASH and HCC. Future work will necessarily involve understanding how hepatic URI expression is upregulated in response to excessive nutrient uptake. URI regulation is an important factor when considering IL-17A inhibitors as therapeutics for HCC, as it is possible that high patient URI levels could overcome IL-17A depletion in humans via the feed-forward loop. However, the efficacy of inhibiting both molecules for the treatment of HCC has not yet been tested in vivo. Clues to the metabolic regulation of URI may be in the role of this protein in inhibiting tryptophan catabolism (4). Finally, as high URI levels have also been shown to be important in other cancers, such as uterine (7) and ovarian (6) cancer, it is possible that URI can also mediate tumorigenesis through other, as yet unknown, pathways.
Acknowledgments
Funding: This work was funded by a James Cook University Rising Star Grant (AAR), Cancer Council New South Wales Project Grant APP1069733 (LH) and a James Cook University Development Grant (LH).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Zhen-Yu Lin (Cancer center, Union hospital, Huazhong University of Science and Technology, Wuhan, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.11.16). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Laursen L. A preventable cancer. Nature 2014;516:S2-3. [Crossref] [PubMed]
- Karin M, Dhar D. Liver carcinogenesis: from naughty chemicals to soothing fat and the surprising role of NRF2. Carcinogenesis 2016;37:541-6. [Crossref] [PubMed]
- Yang H, Gu J, Zheng Q, et al. RPB5-mediating protein is required for the proliferation of hepatocellular carcinoma cells. J Biol Chem 2011;286:11865-74. [Crossref] [PubMed]
- Tummala KS, Gomes AL, Yilmaz M, et al. Inhibition of de novo NAD(+) synthesis by oncogenic URI causes liver tumorigenesis through DNA damage. Cancer Cell 2014;26:826-39. [Crossref] [PubMed]
- Yang S, Wang H, Guo Y, et al. RMP plays distinct roles in the proliferation of hepatocellular carcinoma cells and normal hepatic cells. Int J Biol Sci 2013;9:637-48. [Crossref] [PubMed]
- Theurillat JP, Metzler SC, Henzi N, et al. URI is an oncogene amplified in ovarian cancer cells and is required for their survival. Cancer Cell 2011;19:317-32. [Crossref] [PubMed]
- Wang Y, Garabedian MJ, Logan SK. URI1 amplification in uterine carcinosarcoma associates with chemo-resistance and poor prognosis. Am J Cancer Res 2015;5:2320-9. [PubMed]
- Gstaiger M, Luke B, Hess D, et al. Control of nutrient-sensitive transcription programs by the unconventional prefoldin URI. Science 2003;302:1208-12. [Crossref] [PubMed]
- Djouder N, Metzler SC, Schmidt A, et al. S6K1-mediated disassembly of mitochondrial URI/PP1gamma complexes activates a negative feedback program that counters S6K1 survival signaling. Mol Cell 2007;28:28-40. [Crossref] [PubMed]
- Gomes AL, Teijeiro A, Burén S, et al. Metabolic Inflammation-Associated IL-17A Causes Non-alcoholic Steatohepatitis and Hepatocellular Carcinoma. Cancer Cell 2016;30:161-75. [Crossref] [PubMed]
- Mita P, Savas JN, Ha S, et al. Analysis of URI nuclear interaction with RPB5 and components of the R2TP/prefoldin-like complex. PLoS One 2013;8:e63879 [Crossref] [PubMed]
- Gu FM, Li QL, Gao Q, et al. IL-17 induces AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in hepatocellular carcinoma. Mol Cancer 2011;10:150. [Crossref] [PubMed]
- Hammad LN, Abdelraouf SM, Hassanein FS, et al. Circulating IL-6, IL-17 and vitamin D in hepatocellular carcinoma: potential biomarkers for a more favorable prognosis? J Immunotoxicol 2013;10:380-6. [Crossref] [PubMed]
- Fan JL, Zhang J, Dong LW, et al. URI regulates tumorigenicity and chemotherapeutic resistance of multiple myeloma by modulating IL-6 transcription. Cell Death Dis 2014;5:e1126 [Crossref] [PubMed]
- Chen XW, Zhou SF. Inflammation, cytokines, the IL-17/IL-6/STAT3/NF-κB axis, and tumorigenesis. Drug Des Devel Ther 2015;9:2941-6. [PubMed]
- Bartlett HS, Million RP. Targeting the IL-17-T(H)17 pathway. Nat Rev Drug Discov 2015;14:11-2. [Crossref] [PubMed]
- Umemura A, Park EJ, Taniguchi K, et al. Liver damage, inflammation, and enhanced tumorigenesis after persistent mTORC1 inhibition. Cell Metab 2014;20:133-44. [Crossref] [PubMed]
- Zhu AX, Kudo M, Assenat E, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA 2014;312:57-67. [Crossref] [PubMed]


